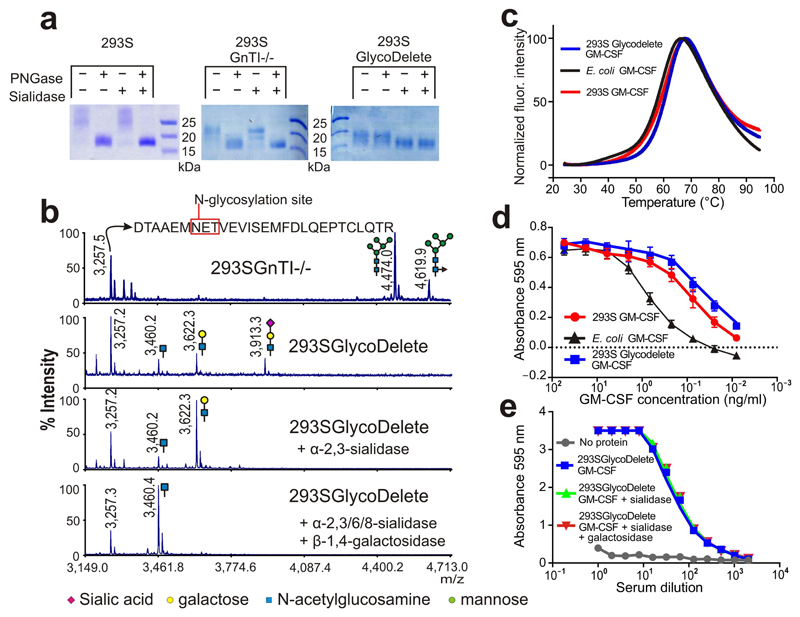
Fig. 2. GlycoDelete glycan characterization.
(a) SDS-PAGE of 293S, 293SGnTI-/- and 293SGlycoDelete GM-CSF samples. Each sample was treated with PNGaseF, sialidase or both enzymes, analysed on an SDS-PAGE gel and stained with coomassie brilliant blue. The non-cropped can be found in Supplementary Fig. 15. (b) MALDI-TOF-MS spectra of GM-CSF samples. Peaks are labeled with their m/z values. The spectrum of the 293SGnTI-/- GM-CSF reveals the presence of Man5GlcNAc2 and fucosylated Man5GlcNAc2 on the glycopeptide containing N37 (top spectrum; left and right glycans, respectively). These glycoforms are absent in GlycoDelete GM-CSF (2nd spectrum). New peaks at m/z values corresponding to HexNAc, Hex-HexNAc and Sia-Hex-HexNAc modified glycopeptides are detected. Spectra of exoglycosidase-digested GlycoDelete GM-CSF N-glycans with α-2,3-sialidase or both a broad spectrum sialidase and β-1,4-galactosidase are shown. These spectra show that N-glycans on GlycoDelete GM-CSF N37 are Neu5Ac-α-2,3-Gal-β-1,4-GlcNAc and Gal-β-1,4-GlcNAc. (c) Thermofluor assay of 293S, 293SGlycoDelete and E. coli produced GM-CSF. We observed similar average (n=3) melting curves for all GM-CSF glycoforms (Tm is approximately 60°C). (d) Bioactivity of 293S and 293SGlycoDelete produced GM-CSF as measured in a TF1 erythroleukemia cell proliferation assay (n=3). E. coli produced GM-CSF serves as a non-glycosylated control sample. The error bars are S.D. Numerical data for this graph are in Supplementary Table 3. (e) ELISA analysis of anti-glycan antibody titers in GlycoDelete GM-CSF immunized rabbit serum. Removal of sialic acid and galactose monosaccharides from the GlycoDelete glycan does not reduce serum antibody recognition. Numerical data for this graph are in Supplementary Table 4.