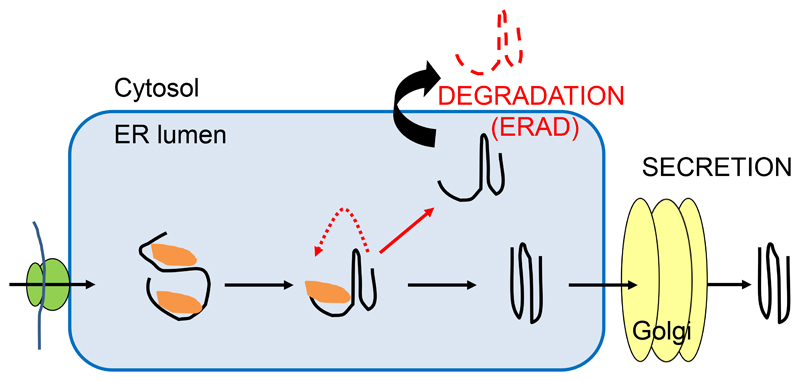
Figure 1. Schematic overview of ERQC processes.
As soon as a polypeptide emerges in the lumen of the ER it faces numerous chaperones (shown in orange) and enzymes that promote folding and prevent aggregation. While proper folded proteins with native conformations exit the ER to the Golgi, terminally misfolded proteins are degraded by ER-associated degradation (ERAD). A critical step in ERQC is the distinction between folding intermediates in productive folding cycles and folding-defective polypeptides in futile folding processes.