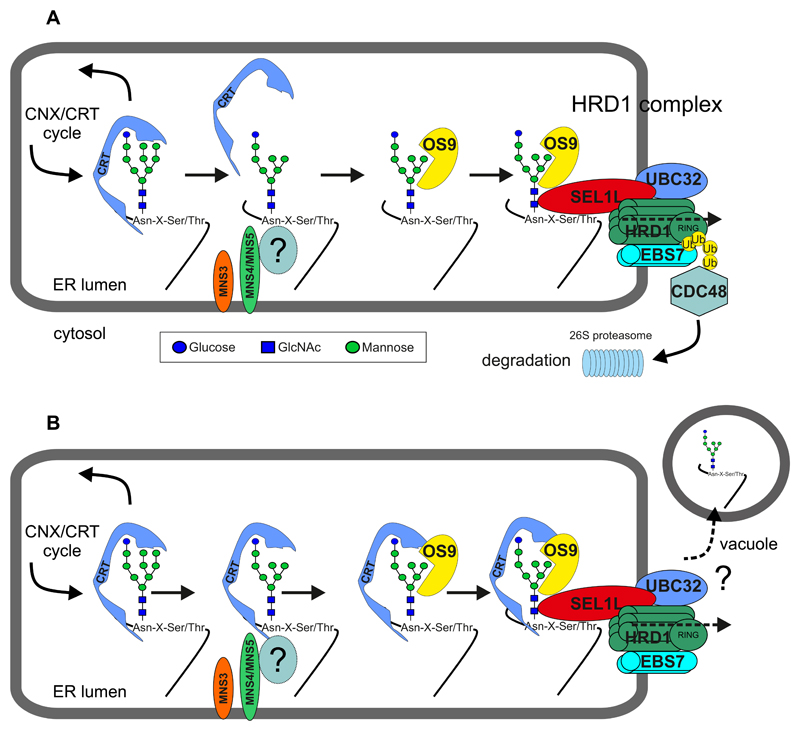
Figure 4. Proposed models for glycan-dependent ERAD via the HRD1 complex.
Terminally misfolded glycoproteins are extracted from the CNX/CRT cycle and sent for degradation by a poorly understood mechanism, which involves the detection of a non-native protein conformation and mannose trimming. The two ER-resident α-mannosidases MNS4 and MNS5 remove the terminal mannose residue from the C-branch and generate the free α1,6-mannose that is recognized by the lectin OS9 in combination with SEL1L. Additional luminal factors like BiP (not shown) may help to direct the misfolded protein to the HRD1 complex. The mannose on the B-branch is removed by MNS3. In contrast to mammals and yeast, this trimming reaction is dispensable for plant ERAD (57). A yet unknown factor (“?”) may act as a folding sensor and assist MNS4/MNS5 in the termination of futile folding attempts. (A) MNS4/MNS5 activity diverts misfolded proteins out of the CNX/CRT cycle leading to dissociation of the lectin. The disposal of misfolded glycoproteins requires the HRD1 complex. HRD1 is implicated in ERAD substrate retro-translocation to the cytosol (presumably by forming a channel) and polyubiquitination (Ub) mediated by the RING domain of HRD1. Extraction of the polyubiquitinated protein and subsequent degradation by the 26S proteasome involves the cytosolic ATPase CDC48 and accessory proteins (not shown). UBC32 associates with the HRD1 complex or is part of the plant DOA10 complex (not indicated here) and involved in the regulation of the ERAD component OS9. EBS7 is involved in the maintenance of HRD1 stability. (B) In the “persistent binding model”, de-mannosylation of the B- and C-branches attenuates GCSII-mediated de-glucosylation leading to enhanced CNX/CRT-binding. The CNX/CRT-bound glycoprotein is escorted to the HRD1 complex. The CNX/CRT-binding is either lost during translocation and degradation following the pathway as shown in (A) or the misfolded glycoprotein is directed to an alternative degradation route involving transport to the vacuole.