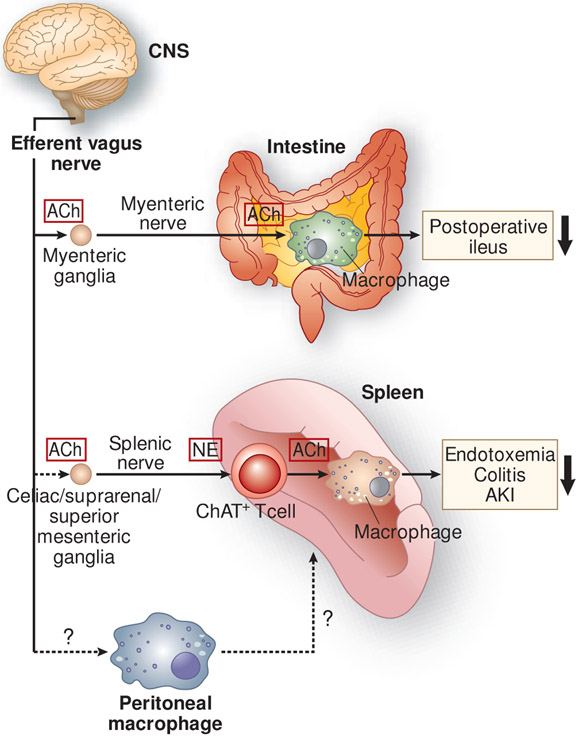
Figure 2.
Anti-inflammatory neural circuits elicited by the stimulation of the efferent vagus nerve. In the cholinergic anti-inflammatory pathway, stimulation of the efferent vagus nerve appears to transmit a signal to the splenic nerve, although a direct connection between the efferent vagus and splenic nerves remains controversial. Norepinephrine (NE), released from splenic nerve terminals, interacts with β2-adrenergic receptors expressed on choline acetyltransferase (ChAT)-positive (acetylcholine-producing) T cells in the spleen, which leads to the release of acetylcholine (ACh) from these cells. Subsequently, ACh binds to α7 nicotinic acetylcholine receptors (α7nAChRs) expressed on macrophages, resulting in the suppressed production of pro-inflammatory cytokines by macrophages and suppressed inflammation. This pathway has been demonstrated in many inflammatory disease models, including endotoxemia, colitis, and acute kidney injury (AKI). Another anti-inflammatory circuit involves a direct interaction between ACh released from the myenteric nerve and α7nAChRs expressed on muscularis macrophages of the intestine in postoperative ileus. Vagus nerve stimulation (VNS) causes phenotypic changes in peritoneal macrophages toward anti-inflammation, and adoptive transfer of these macrophages confers protection from AKI, which requires the spleen. The mechanism underlying changes in the phenotype of peritoneal macrophages after VNS and interaction of peritoneal macrophages with immune cells in the spleen remains unclear. CNS, central nervous system.