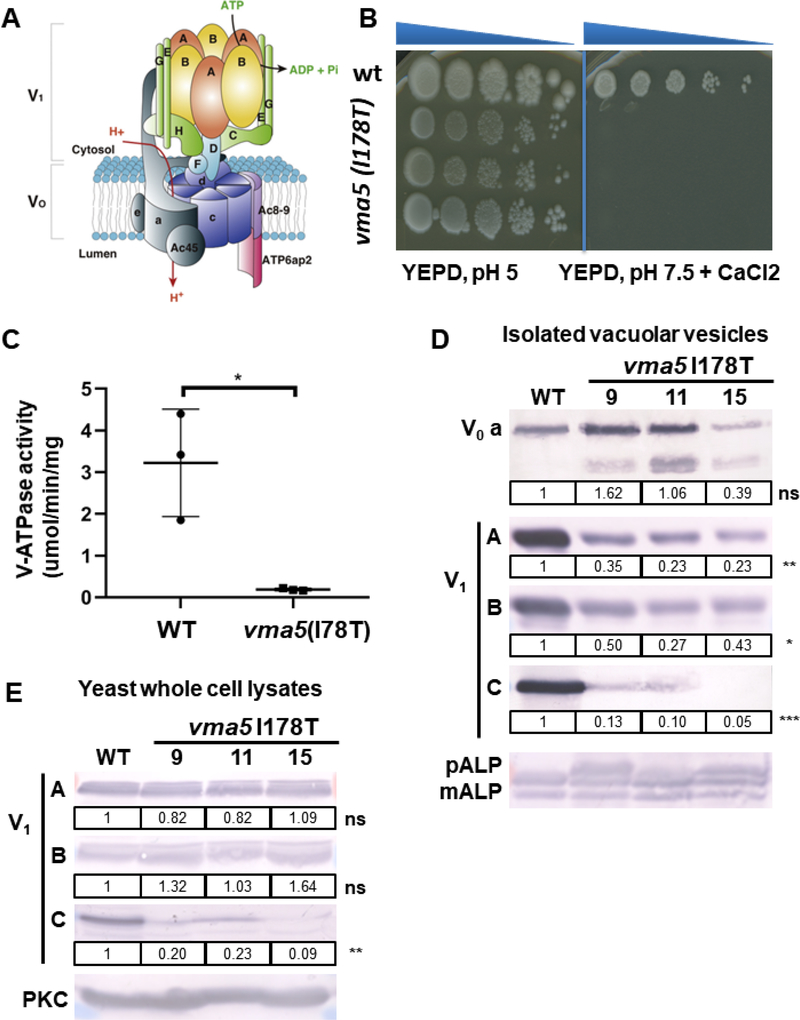
Figure 3: vma5 (I178T) mutants display a growth defect and reduced V-ATPase activity.
(A) Structure of V-ATPase (modified from12) including a peripheral domain (V1) important for ATP hydrolysis and an integral domain (V0) responsible for proton translocation. The V1 domain includes A and B subunits in a hexameric arrangement connected to the V0 domain via peripheral stalks comprising subunits C, E, G, H and the N-terminal domain of subunit a. The V0 domain includes a ring of proteolipids (c) adjacent to subunits a and e. (B) 3 independent isolates (9, 11, 15) containing the I178T vma5 mutation fail to grow on YEPD, pH 7.5, CaCl2 plates, whereas no growth loss was evident on YEPD, pH 5 plates. (C) Mean concanamycin A-sensitive V-ATPase activity in vacuolar vesicles from WT and vma5 I175T mutants (n=3 for each, error bars represent S.E.) (D) The levels of both membrane-bound (vacuolar V0 subunit a, Vph1) and peripheral V1 subunits (A, B, and C) were visualized by immunoblot and normalized to mature ALP (mALP), a vacuolar membrane protein. mALP runs as 2 bands as in the wild-type sample. If V-ATPase activity and therefore, vacuolar proteolytic activity, is reduced, a third higher molecular weigth pro-ALP band (pALP) appears, which is present in the mutants. The levels of Vph1 were similar in vacuolar vesicles prepared from 3 different mutant strains compared to WT (ns, P=0.94), but the level of the Vma5 protein was reduced (***, P ≤ 0.001), as were the levels of the V1 subunits, A (**, P ≤ 0.01) and B (*, P ≤ 0.05).
(E) V1 subunits (A, B, and C) were measured via immunoblot in yeast whole cell lysates of WT and 3 different mutant strains and normalized to PKC as loading control. Levels of the C subunit are reduced in the mutant strains (**, P ≤ 0.01).