FIGURE 1.
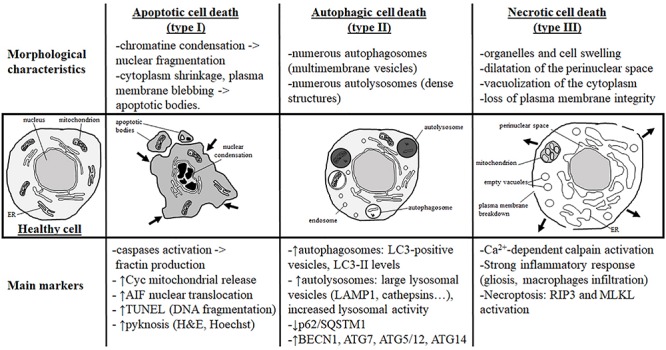
Morphological characteristics and most currently used markers of the three main types of cell death. Analysis of the dying cell ultrastructure is the most reliable method to identify the cell death type. The hallmark of apoptotic cell death (or type I) is a modification of the nuclear structure with presence of chromatin condensation and nuclear fragmentation. The cytoplasm shrinks and plasma membrane deforms until it forms apoptotic bodies that will be removed by phagocytic cells. Molecular markers of apoptosis are caspases (mainly caspase-3) and sometimes cytochrome c (Cyc) mitochondrial release in the cytosol. As a marker of caspase-independent apoptosis, nuclear translocation of AIF (Apoptosis-Inducing Factor) is mainly used. Pyknosis (condensation) of the nucleus shown by H&E (hematoxylin/eosin) or Hoechst stainings is also a main feature of apoptosis. TUNEL (Terminal deoxynucleotidyl transferase dUTP nick end labeling) is a staining revealing DNA fragmentation used as a marker of apoptosis with caution if not complemented with a cleaved-CASP3 immunostaining. Autophagic cell death (or type II) is mainly characterized by the presence of numerous autophagosomes (multimembrane vesicles containing intact cytoplasmic material such organelles) and autolysosomes (electron dense structure due to material at different stage of degradation). To conclude on autophagy flux with biochemical markers, it is necessary to study both autophagosome formation and autolysosome degradation. In autophagic cell death, both processes have to be enhanced. Autophagosome presence is reflected by the number of LC3-positive vesicles or by LC3-II level of expression on immunoblot. When autophagic degradation activity is enhanced, vesicles positive for lysosomal markers [such as LAMP1 (lysosomal-associated membrane protein 1) or cathepsins] are increased in number and size since autolysosomes are larger than primary lysosomes. The decrease in p62/SQSTM1 (Sequestosome-1), an autophagosome cargo protein degraded selectively by autophagy, reveals enhanced autophagy. In some cases, the level of autophagy-related (ATG) proteins such as BECN1 (BECLIN1/ATG6), ATG7, ATG5/ATG12, or ATG14 is increased. In necrotic cell death (or type III), ultrastructural changes are mainly cytoplasmic with organelles and cell swelling and presence of empty vacuoles. Perinuclear space is also dilated. Plasma membrane integrity can be finally lost, inducing a strong inflammatory response. Very few tools are available to identify necrotic cell death and mainly indirect markers are used such as strong inflammation response or Ca2+-dependent activation of calpains [mainly suggested by the production of a 145- to 150-kDa calpain-dependent cleavage of spectrin (fodrin)]. Necroptosis type of necrosis could be investigated by RIP3/RIPK3 (Receptor-interacting serine/threonine-protein kinase) or MLKL (mixed lineage kinase domain-like) expression and activation.
