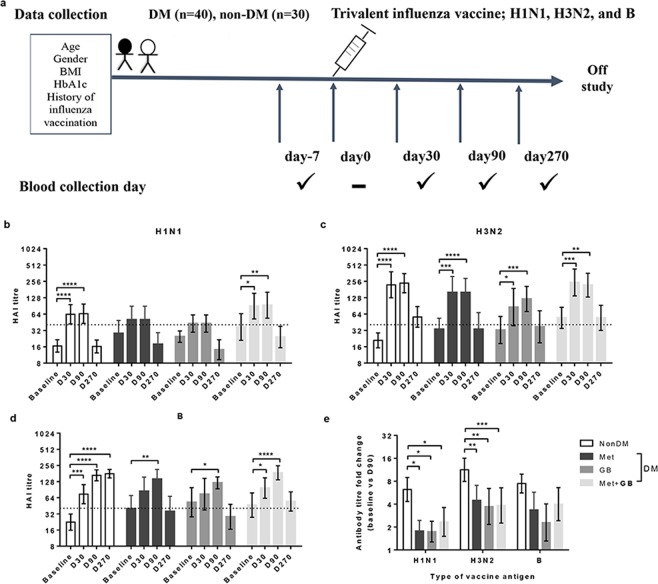
Figure 1.
Seasonal vaccination with TIV elicited sero-protection for all groups, but all treated DM groups showed significantly decreased responses against H1N1 and H3N2, in addition to delaying and reducing haemagglutination inhibition (HAI) persistency against influenza B antigen in metformin-DM and glibenclamide-DM. (a) Study design indicating the sample groups and time of blood collection before and after seasonal vaccination with TIV (schematic). Blood samples were collected from Thai individuals with and without T2DM at baseline (day -7) and post-vaccination with TIV (day 0). Antibody responses were evaluated by the HAI assay at baseline, as well as 30, 90, and 270 days post-vaccination against influenza (b) H1N1, (c) H3N2, or (d) B-antigen and compared with non-DM individuals and T2DM patients prescribed metformin (Met; n = 12) or glibenclamide (GB; n = 10), or a combination of the two drugs (Met + GB; n = 18). The dotted line represents the protective threshold HAI titre of 40. (e) HAI antibody titre fold-change (baseline vs. D90) against influenza H1N1, H3N2, or B-antigens compared among all four groups. Comparison of percentage (%)Statistical analyses were undertaken using one-way (b–d) or two-way (e) ANOVA. Horizontal lines represent the geometric mean with 95% CI. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.