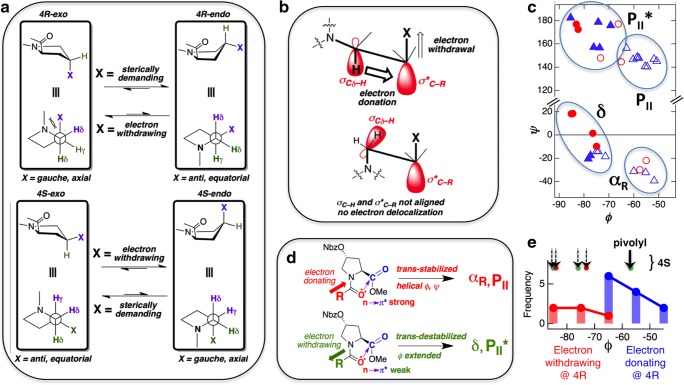
Fig. 4.
a Schematic showing the effects of a sterically demanding or an electron-withdrawing substituent at position 4 of Pro. b A schematic depicting the gauche effect—a stereoelectronic effect that stabilizes the gauche conformer when an electron-withdrawing group is present at position 4 of Pro. c Ramachandran plot for γ-substituted L-Pro analogs (triangle trans; circle cis; filled symbols represent endo while open symbols represent exo). More details about the structures used are given in Table S2. d A schematic depicting the effect of electron-withdrawing versus electron-donating substitutions at the N-cap position of 4-nitrobenzoatehydroxyproline on backbone dihedral angles, mediated by n → π* interactions. The nitrobenzoate group (NBz) is an electron-withdrawing substituent and hence it favors the exo ring pucker when substituted at the 4R position and endo ring pucker when substituted at the 4S position. e Distribution of dihedral angle ϕ in 4R-substituted proline, separated in two groups: one with electron-donating (blue) and the other with electron-withdrawing (red) N-cap. The corresponding 4S variants are also shown (arrows on the top) where all except the pivolyl N-cap show extended ϕ