Abstract
Unlike sex steroids, mineralocorticoids have attracted limited attention in ovarian physiology. Recent studies on primates have indicated possible local synthesis and action of mineralocorticoids in the ovary. Here, we examined developmental changes in the levels of mineralocorticoids and expression of genes encoding their biosynthetic enzymes and receptor in the bovine ovary. The follicles and corpora lutea (CL) were collected from F1 heifers. Expression levels of 21α-hydroxylase (CYP21A2), 11β-hydroxylase-1 (CYP11B1), and the mineralocorticoid receptor (NR3C2) in granulosa cells (GC), thecal layers (TL), and CL tissues were quantified by real-time PCR, whereas mineralocorticoids in the follicular fluid were measured by enzyme immunoassay (EIA). TL and GC expressed CYP21A2 and NR3C2, whereas CYP11B1 was expressed at very low or undetectable levels. The expression levels of these genes were not significantly different among small/large and healthy/atretic follicles but were higher in TL than in GC. CYP21A2 and NR3C2 were expressed in all CL stages with higher expression observed in the mid-stage. CYP11B1 expression was only apparent in the mid-stage CL. Aldosterone was detected in all follicles, and its concentration was not significantly different among the follicular groups. In paired large-healthy/atretic follicles, the concentration of deoxycorticosterone, a precursor of aldosterone, was approximately ten-fold higher than that of aldosterone and not significantly different between healthy and atretic follicles. In conclusion, the presence of mineralocorticoids and expression of NR3C2 in the bovine follicle together with the developmental change in the expression of CYP21A2, CYP11B1, and NR3C2 in the CL suggest possible endocrine/paracrine/autocrine roles of mineralocorticoids in the bovine ovary.
Keywords: Aldosterone, Corpus luteum, Follicle, Mineralocorticoid, Mineralocorticoid receptor
Mineralocorticoids belong to the C21 corticosteroids produced in the adrenal cortex and play a crucial role in controlling electrolyte and fluid balance in target organs such as the kidney. Mineralocorticoids are synthesized from progesterone (P4) through a series of reactions. In the first step, mediated by 21-hydroxylase (CYP21A2), P4 is converted to deoxycorticosterone (DOC), an active but less potent mineralocorticoid. Subsequently, DOC is converted to corticosterone through 11β-hydroxylation, which in turn, through 18-hydroxylation and 18-methyl oxidation, is converted into the prime mineralocorticoid – aldosterone (Aldo). In primates and rodents, the last two reactions are mediated by 11β-hydroxylase-1 (CYP11B1) and 11β-hydroxylase-2 (CYP11B2), respectively [1,2,3,4,5], whereas in livestock species such as cattle, sheep, and pigs, CYP11B1 mediates both these processes [6,7,8]. Therefore, the expression of these enzymes is a prerequisite for local mineralocorticoid synthesis in an organ.
Mineralocorticoids are classically thought to be produced only in the adrenal cortex; however, evidence indicating extra-adrenal mineralocorticoid production has accumulated in recent years [9]. Likewise, the ovary has been shown to produce mineralocorticoids in various species. In macaques, cultured granulosa cells (GC) exposed to human chorionic gonadotropin (hCG) express CYP21A2 and produce DOC [10]. In cattle, GC and theca cells were shown to express functional CYP11B1 [11]. In humans, concentrations of Aldo and its precursor corticosterone in preovulatory follicles stimulated by an ovulatory dose of hCG are higher than those in the plasma [12]. In addition, luteinized GC have been shown to express CYP21A2 [13]. In addition, the presence of the mineralocorticoid receptor (NR3C2) has been reported in the ovary of rats [14, 15], macaques [10], cattle [16], and humans [12, 13]. Altogether, these results indicate that the ovary is constantly exposed to circulating and possibly locally synthesized mineralocorticoids. Despite these observations, only a few studies have examined the effects of mineralocorticoids on ovarian functions. In macaques, Fru and co-workers demonstrated that locally synthesized DOC acts through NR3C2 to stimulate P4 production in cultured granulosa cells [10]. Recently, we showed that Aldo enhances bovine oocyte maturation [17]. These results indicate that mineralocorticoids act not only as endocrine factors but also as autocrine/paracrine factors to regulate ovarian physiology.
To our knowledge, developmental changes in the expression of mineralocorticoid biosynthetic enzymes and NR3C2 have not been reported in the bovine follicle and corpus luteum (CL). Therefore, in this study, we examined the expression levels of CYP21A2, CYP11B1, and NR3C2 in bovine follicles and CL of various physiological states and developmental stages. Moreover, we examined the concentrations of the corticosteroids DOC, Aldo, and cortisol together with those of the prime ovarian steroids estradiol (E2) and P4 in the follicular fluid collected from healthy and atretic follicles.
Materials and Methods
Sample collection and preparation
Pairs of ovaries were collected from Holstein-Japanese Black F1 heifers at a local slaughterhouse. The ovaries were kept on ice and transported to the laboratory within 30 min of slaughter. The ovaries were macroscopically inspected, and the number and size of the follicles and corpora lutea (CL) were recorded. The follicular fluid (FF) was aspirated from the follicles using a disposable syringe fitted with a 20G needle, and its weight was recorded [18]. The aspirated follicles and CL were dissected from ovaries and stored in RNAlater (Ambion, Austin, TX, USA) and kept at −30°C until further preparation of the samples.
Granulosa cells (GC) were carefully scraped off from follicular walls using a spatula, pelleted, and lysed with TRIzol Reagent (Thermo Fisher Scientific, Waltham, MA, USA). The follicular walls were further cleared of the remaining GC and stroma to obtain thecal layers (TL) [18]. The TL were cut into small pieces and kept in TRIzol Reagent at −30°C until RNA extraction. The adrenal gland and kidney were also harvested from a Holstein cow to be used as positive controls in mRNA analysis and kept in RNAlater at −30°C until RNA extraction.
Corpus luteum and follicle classification
The CL were macroscopically examined for color and vascularity and further classified into four stages (luteal phase: stages I–III; follicular phase: stage IV) according to the criteria reported by Ireland et al. [19]. Diameters of the follicles were estimated based on the weight of the follicular fluid using the equation y = 12.96x0.31, where y is the diameter of the follicle (mm) and x is the weight of the follicular fluid (g) [20]. Based on the relative concentrations of estradiol (E2) and progesterone (P4) in the follicular fluid, all follicles were initially classified as healthy (E2/P4 ≥ 1) and atretic (E2/P4 < 1) as reported by Nishimoto et al. [21]. The follicles were further classified into seven groups based on the diameter (small: < 8.5 mm or large: ≥ 8.5 mm) and stage and status of the accompanying CL and follicles, respectively [21]. Accordingly, large healthy follicles were classified either as dominant (DF) or preovulatory follicles (POF), whereas large atretic follicles were classified as early atretic (EAF), mid-atretic (MAF), or late atretic follicles (LAF). Small follicles were classified as small growing follicles (SGF) or small atretic follicles (SAF) (Table 1).
Table 1. Follicular classification criteria.
| Follicular class | Size (mm) | E2/P4 (w/w) | Accompanying follicles | Corpora lutea (CL) stage |
|---|---|---|---|---|
| Small atretic (SAF) | < 8.5 | < 1 | DF/POF | I–IV |
| Small growing (SGF) | < 8.5 | ≥ 1 | No DF/POF | I–III |
| Dominant (DF) | ≥ 8.5 | ≥ 1 | SAF/MAF | I–III |
| Preovulatory (POF) | ≥ 8.5 | ≥ 1 | LAF | IV |
| Early atretic (EAF) | ≥ 8.5 | < 1 | SGF, no DF/POF | II–III |
| Mid-atretic (MAF) | ≥ 8.5 | < 1 | DF | II–III |
| Late atretic (LAF) | ≥ 8.5 | < 1 | EAF/POF | III–IV |
Total RNA extraction and cDNA synthesis
Total RNA was extracted from GC, TL, CL, and positive control samples using TRIzol Reagent according to the manufacturer’s instruction. Pelleted RNA was dissolved in THE RNA Storage Solution (Thermo Fisher Scientific) at a concentration of 1 µg/µl and kept at −80°C. Removal of contaminating genomic DNA and cDNA synthesis were performed by using the QuantiTect Reverse Transcription kit (Qiagen, Hilden, Germany) with 2 µg RNA per sample according to the manufacturer’s instruction.
Quantitative PCR
The levels of mRNAs encoding CYP21A2, CYP11B1, NR3C2, 11β-hydroxysteroid dehydrogenase type 2 (HSD11B2), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were quantified by real-time quantitative PCR using LightCycler Nano (Roche Diagnostics, Mannheim, Germany) and FastStart Essential DNA Green Master (Roche). The primers were designed using the National Centre for Biotechnological Information (NCBI) primer designing tool Primer-BLAST [22] based on the reported bovine sequences (Table 2), and were purchased from Sigma. The amplification program consisted of an initial activation for 10 min at 95°C, followed by 45 cycles of denaturation at 95°C for 10 sec, annealing at 60°C for 10 sec, and extension at 72°C for 15 sec. The authenticity of the PCR products was verified by monitoring the melting curve and the size of the PCR products. All primer pairs produced a clear single peak melting curve profile and amplicons at the expected size. A positive control (adrenal gland for CYP21A2, CYP11B1, and GAPDH; kidney for NR3C2 and HSD11B2) and a negative control (dH2O) were included in each PCR run. Amplifications were performed in duplicates. For all quantifications, the intra- and inter-assay coefficients of variation (CV) were less than 10% and 15%, respectively. For normalization of mRNA data, GAPDH was used as an internal control. Relative gene expression was calculated by the 2–ΔCt method.
Table 2. Primers used for quantitative real-time PCR.
| Gene | Primer | Sequence (5ʹ-3ʹ) | GenBank no. | Position |
|---|---|---|---|---|
| CYP21A2 | Forward | CCTGGAGCTGTTCGTGGTG | NM_174639.1 | 1442–1460 |
| Reverse | GCTGGACCTTGAGGTTGACA | NM_174639.1 | 1544–1563 | |
| CYP11B1 | Forward | CCATCGAAGCCAGCACCTTA | NM_174638.3 | 592–611 |
| Reverse | CTGGGCACAAACATGAGCTG | NM_174638.3 | 711–730 | |
| NR3C2 | Forward | CCGTACCCACGGAGCAGTC | NM_001191349.2 | 216–235 |
| Reverse | CTTGCTGGAGGCAAGGGAGT | NM_001191349.2 | 98–116 | |
| HSD11B2 | Forward | CGAGCACTTGAATGGGCAGTT | AF074706 | 1033–1053 |
| Reverse | CCTGGGTAATAGCGGCGGAGT | AF074706 | 1135–1155 | |
| GAPDH | Forward | GCGCCAAGAGGGTCATCATC | NM_001034034.2 | 409–428 |
| Reverse | AGTCCCTCCACGATGCCAAA | NM_001034034.2 | 567–586 |
Steroid assay
Commercial enzyme immunoassay (EIA) kits were obtained from Cayman Chemical Company (Ann Arbor, MI, USA) for measuring E2, P4, Aldo, and cortisol, and from MyBioSource (San Diego, CA, USA) for measuring DOC. For measuring E2, P4, and cortisol, the follicular fluid samples were diluted with EIA buffer at dilution factors of 1:100–1:10,000, and EIAs were performed according to the manufacturers’ instructions. Undiluted follicular fluid samples were used to measure Aldo, whereas, for DOC measurement, the samples were diluted with PBS at a dilution factor of 1:5. In order to construct standard curves for Aldo assays, a steroid-free follicular fluid was used for standard preparations. For this purpose, steroids were removed from pooled follicular fluid by incubating the fluid with activated charcoal (Sigma) according to the manufacturer’s instructions. E2, P4, and Aldo were measured in all samples, whereas DOC and cortisol were measured only in paired samples. The intra- and inter-assay CV were 6.1% and 9.8% for E2, 8.0%, and 17.1% for P4, and 5.2% and 7.3% for Aldo, respectively. In paired samples, the intra-assay CV for DOC and cortisol were 9.3% and 3.4%, respectively.
Statistical analysis
Data analysis was performed by the software R 3.5.2 for Windows (R Studio Team, 2016). After failing the normality test, the difference among groups was examined by the Kruskal test, followed by the Wilcoxon rank-sum test. In order to examine the differences between the paired follicular samples, the Wilcoxon signed-rank test was used. The results of data analyses were expressed as medians (interquartile range), and differences between the groups were considered significant at P < 0.05. The relationship between the concentration of cortisol and expression of HSD11B2 was analyzed using Spearman’s rank correlation coefficient.
Results
Concentrations of E2, P4, and Aldo in the follicular fluid of classified follicles
Follicular fluid samples from 56 classified follicles belonging to 35 animals were analyzed for steroid concentrations. There were clear and significant differences among follicular groups in E2 and E2/P4 ratio except between DF and EAF. There was no significant difference in the P4 concentration, although it substantially increased in LAF (Table 3). The median concentration of Aldo in all the follicular fluid samples was 95.2 (67.5–158.5) pg/ml, and, at most, three orders of magnitude lower than that of its precursor P4. Moreover, the Aldo levels were not significantly different among the follicular categories (Table 3).
Table 3. Characteristics of follicles.
| Follicular category (n) | Size | E2 | P4 | E2/P4 ratio | Aldo |
|---|---|---|---|---|---|
| (mm) | (ng/ml) | (ng/ml) | (pg/ml) | ||
| SGF (8) | 7.9 (7.6–8.0) a | 61.1 (23.6–97.0) a | 23.0 (14.4–28.1) a | 3.3 (2.0–4.2) a | 105.7 (81.4–118.2) |
| DF (7) | 13.4 (12.5–13.9) b | 90.0 (53.1–111.5) a | 46.7 (41.8–77.3) a,b | 1.3 (1.1–3.7) a,d | 80.2 (40.0–113.9) |
| POF (8) | 15.9 (14.8–17.3) b | 773.4 (511.1–1471.5) b | 60.8 (48.0–68.5) a,b | 15.4 (9.9–21.5) b | 127.8 (91.8–161.4) |
| SAF (8) | 7.9 (7.3–8.1) a | 3.0 (2.4–5.7) c | 258.0 (127.7–340.7) b | 0.0 (0.0–0.0) c,d | 196.3 (114.3–216.5) |
| EAF (8) | 13.4 (11.1–13.9) b | 22.3 (15.4–36.4) a | 113.4 (33.5–241.3) a,b | 0.4(0.1–0.7) d | 123.7 (66.9–182.7) |
| MAF (9) | 13.2 (11.1–13.5) b | 3.0 (2.6–5.6) c | 60.1 (30.7–137.0) a,b | 0.0 (0.0–0.2) c,d | 71.6 (67.4–94.0) |
| LAF (8) | 12.6 (11.3–13.7) b | 1.8 (1.4–2.4) c | 424.9 (190.5–589.0) b | 0.0 (0.0–0.0) c | 89.3 (41.8–153.4) |
All the values are shown as medians (interquartile range). Values with different superscripts within a column are significantly different at P < 0.05. SGF, small growing; DF, dominant; POF, preovulatory; SAF, small atretic; EAF, early atretic; MAF, mid-atretic; LAF, late atretic.
Gene expression of CYP21A2, CYP11B1, and NR3C2 in the classified follicles
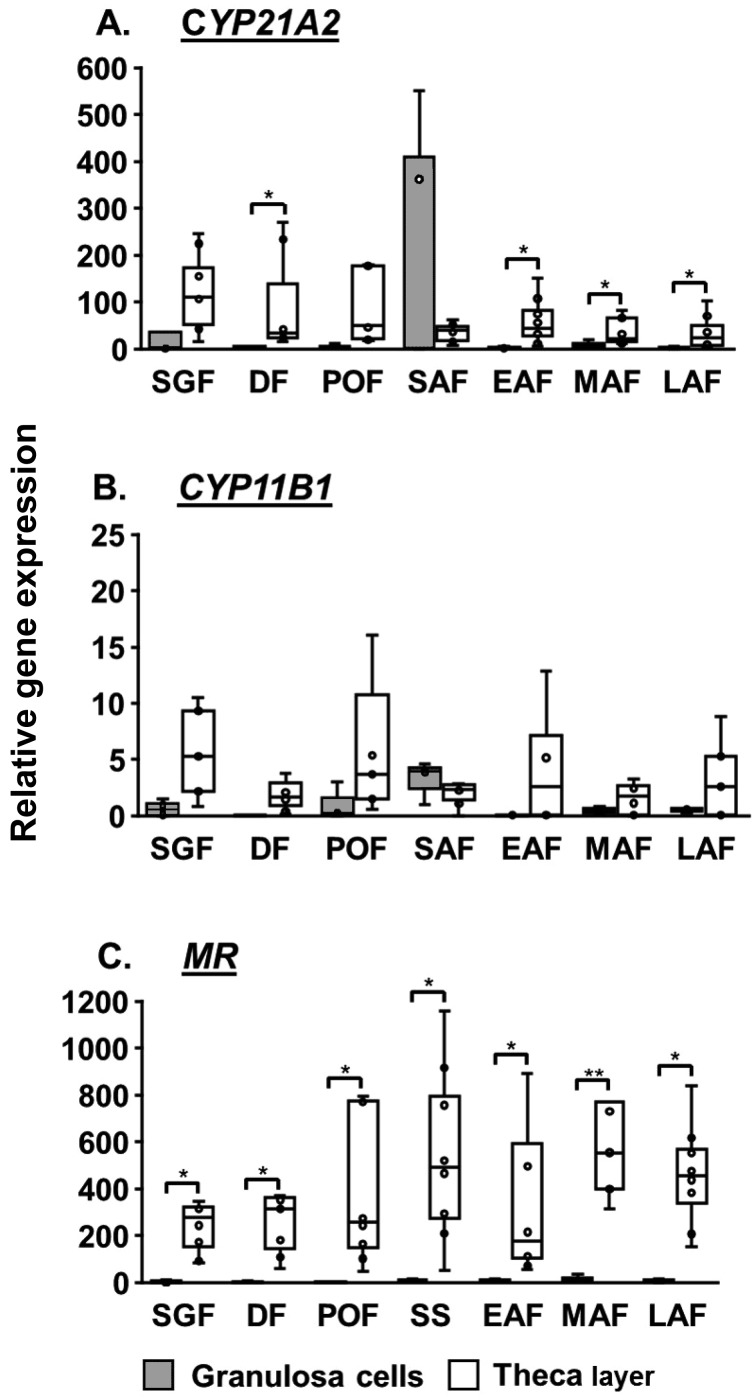
CYP21A2 was expressed in the TL and GC (Fig. 1A). The expression levels were not significantly different among the follicular classes but were much higher in the TL than in the GC. The expression of CYP11B1 was relatively lower and indiscernible in some of the GC and TL follicles (Fig. 1B). Both the TL and GC expressed NR3C2, and the expression was higher in the TL than in the GC. No significant difference was observed among follicular classes in the expression of NR3C2 in the GC and TL (Fig. 1C).
Fig. 1.
Relative expression of CYP21A2 (A), CYP11B1 (B), and NR3C2 (C) in bovine granulosa cells (GC) and theca layer (TL) during follicular maturation and atresia. SGF, small growing follicle; DF, dominant follicle; POF, preovulatory follicle; SAF, small atretic follicle; EAF, early atretic follicle; MAF, mid-atretic follicle; LAF, late atretic follicle. Values are shown as medians and interquartile ranges with bars displaying minimum and maximum values. * P < 0.05, ** P < 0.01.
Gene expression of CYP21A2, CYP11B1, and NR3C2 in the CL
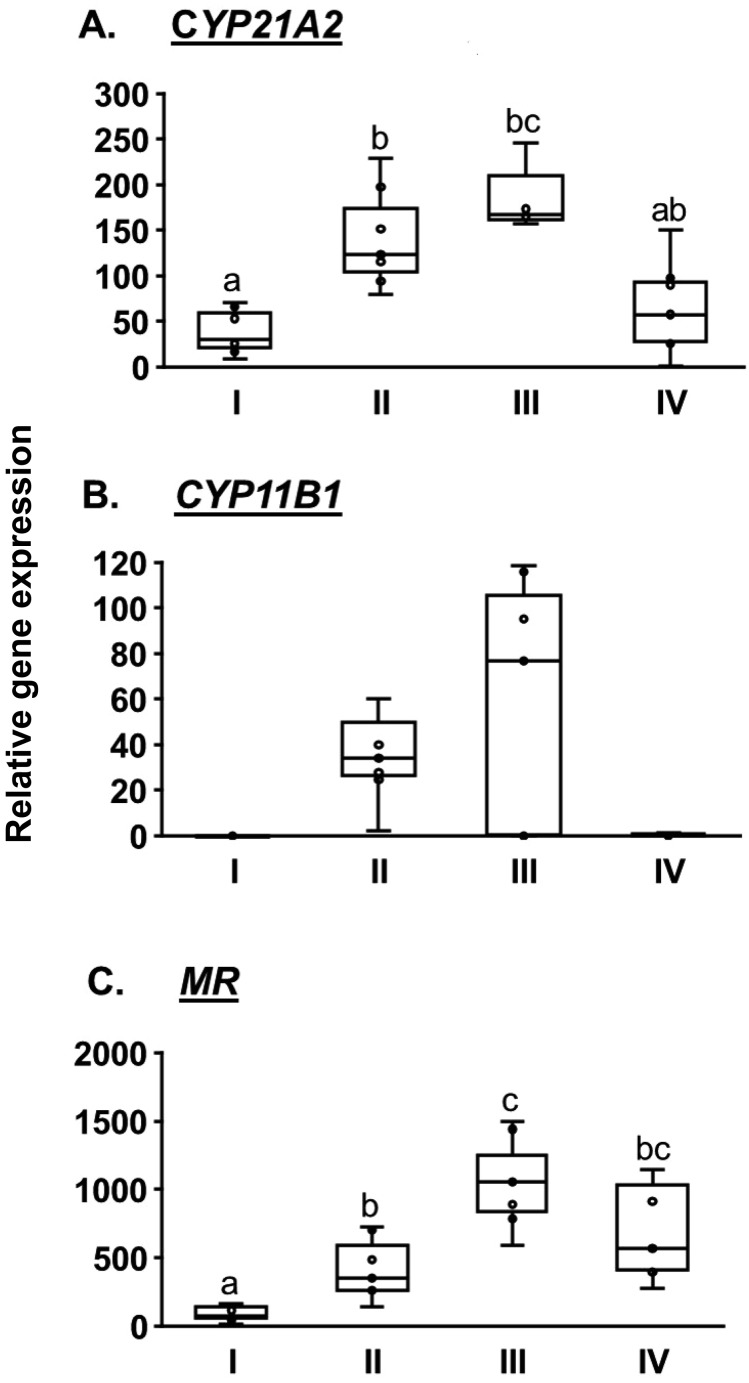
Altogether, 28 CL from 28 animals were analyzed for gene expression. The expression pattern of CYP21A2 followed the production profile of P4, with the highest expression at stage III and a rapid decrease at stage IV (Fig. 2A). The expression levels at stage II and III were significantly higher than at stage I (P < 0.05). The expression levels of CYP11B1 were undetectable at stages I and IV. The levels increased at stages II and III, although no significant difference was observed among the stages (Fig. 2B). The expression of NR3C2 increased at stage II and reached the highest value at stage III, then declined at stage IV. The expression was significantly higher at stages II–IV than at stage I (P < 0.05; Fig. 2C).
Fig. 2.
Relative expression of CYP21A2 (A), CYP11B1 (B), and NR3C2 (C) in bovine corpora lutea (CL) classified into four developmental stages (I–IV). Values are shown as medians and interquartile ranges with bars displaying minimum and maximum values. Different letters indicate significant differences (a, b, c P < 0.05).
Concentrations of DOC, Aldo, and cortisol in large paired healthy and atretic follicles
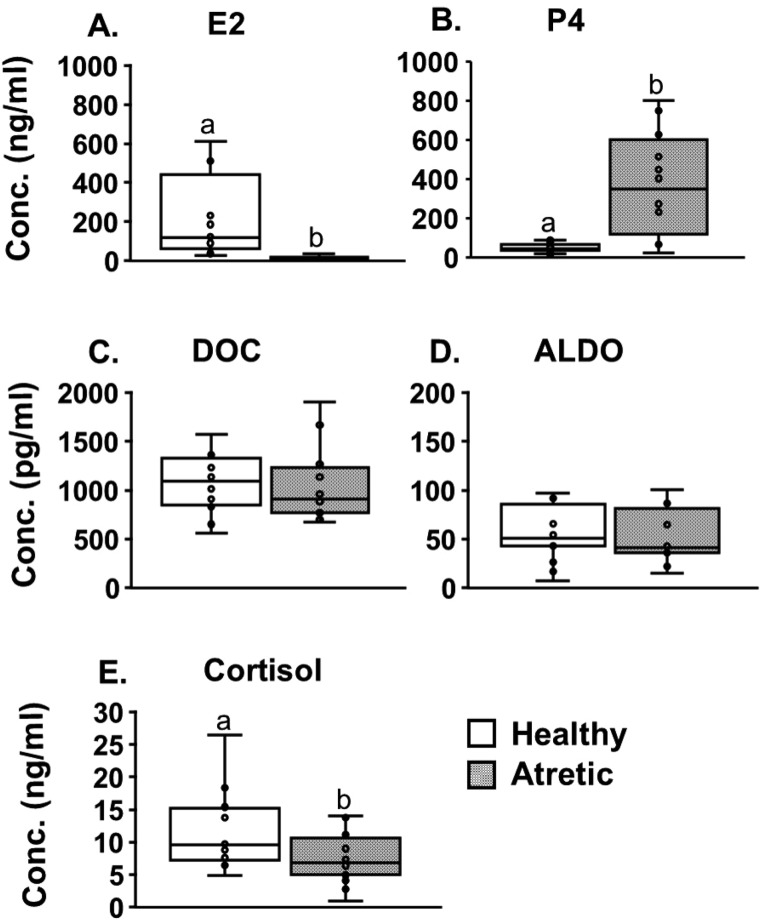
We compared the concentrations of steroids between large healthy (DF and POF) and atretic (MAF and LAF) follicles collected in pairs from 14 animals. Despite apparent differences in the concentrations of E2 and P4 (P < 0.01), no difference was observed in the concentrations of Aldo and DOC. In contrast, the concentration of cortisol was significantly lower (P < 0.05) in the atretic follicles (Fig. 3). The concentration of Aldo in these follicles was approximately 10- and 1000-fold lower than those of its precursors DOC and P4, respectively, and nearly 150-fold lower than that of cortisol.
Fig. 3.
Concentrations (Conc.) of estradiol (E2) (A), progesterone (P4) (B), deoxycorticosterone (DOC) (C), aldosterone (Aldo) (D), and cortisol (E) in the follicular fluid collected from paired healthy and atretic follicles. Values are shown as medians and interquartile ranges with bars displaying minimum and maximum values. Different letters indicate significant differences (a, b P < 0.05, a, c P < 0.01).
Relationship between the concentration of cortisol and gene expression of HSD11B2
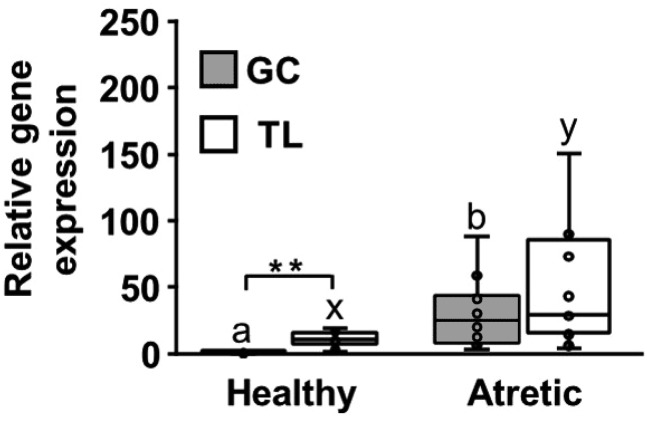
The GC and TL of the paired follicles were examined for expression of HSD11B2, the enzyme that converts cortisol to cortisone. The gene expression was significantly higher in the atretic follicles than in the healthy follicles in both GC and TL (P < 0.05) (Fig. 4). Although the gene expression was significantly higher in TL than in GC of the healthy follicles (P < 0.01), the difference was not significantly different from that observed in atretic follicles. The follicular cortisol concentration strongly and negatively correlated with HSD11B2 expression in TL (ρ = −0.69, P < 0.001).
Fig. 4.
Relative expression of HSD11B2 in bovine granulosa cells (GC) and theca layer (TL) from paired large (˃ 10 mm) follicles (healthy and atretic) within an animal. Values are shown as medians and interquartile ranges with bars displaying minimum and maximum values. Different letters within the same tissue indicate significant differences (a, b; x, y P < 0.05). ** P < 0.01.
Discussion
In cattle, sheep, and pigs, mineralocorticoids are synthesized from P4 through a series of reactions mediated by CYP21A2 and CYP11B1. Therefore, the expression of these enzymes is a prerequisite for de novo synthesis of mineralocorticoids in the bovine follicle and CL. In this study, we have shown that the bovine follicle expresses CYP21A2. However, the expression of CYP11B1 was much lower than that of CYP21A2 and hardly discernible in some follicular samples.
The follicles in humans [13], macaques [10], cattle [11], horses [23], and mice [24] have been shown to express the mineralocorticoid biosynthetic enzymes; however, the expression pattern varied among these species. In macaques, GC primed with FSH expressed CYP21A2, whereas CYP11B1 and CYP11B2, the enzymes mediating the final steps of Aldo synthesis in primates, were undetected before and after an ovulatory stimulus with hCG [10]. Similarly, human GC primed with FSH and exposed to hCG expressed CYP21A2 [13]. Conversely, an ovulatory stimulus with LH/hCG induced the expression of CYP11B1 in murine ovaries [24]. Recently, Amweg et al. (2017) demonstrated the expression of CYP11B1 in the bovine granulosa and theca cells harvested from small (< 5 mm), medium (5–10 mm), and large (> 10 mm) non-atretic follicles [11]. The authors also reported that cultured follicular walls of large antral follicles produced a substantial amount of cortisol, and that ACTH stimulated its synthesis. The synthesis was suppressed by metyrapone, an inhibitor of CYP11B1 [11, 25]. In these studies, neither 11-deoxycortisol, the immediate precursor of cortisol, nor serum, which may contain 11-deoxycortisol, was added to the medium. Thus, these results indicate that the bovine follicle is capable of synthesizing cortisol from de novo synthesized P4. The reason why CYP11B1 could not be detected in some follicular samples is not known. However, the CYP11B1 expression reported by Amweg et al. (2017) may also contain very low levels of gene expression as indicated by the large error bars (SD appeared to be far larger than mean values in some groups) [11].
In the present study, despite the significant difference in size and physiological status among the follicular categories, no significant difference was observed in the gene expression of CYP21A2 and CYP11B1. This lack of difference is due to significant variations in gene expression, as observed by Amweg et al. (2017) [11]. Likewise, Aldo concentrations in the follicular fluid varied substantially and did not differ among the follicular categories. These results imply that the concentrations of Aldo in the follicular fluid are not affected by the status of follicles but by the circulating levels of Aldo. To verify this hypothesis, we collected healthy and atretic follicles in pairs from 14 cows. While E2 and P4 levels were different between the healthy and atretic follicles, DOC and Aldo levels did not differ between these follicles, indicating that follicular levels of DOC and Aldo are not affected by the physiological status of the follicle.
To our knowledge, only one study has reported mineralocorticoid concentrations in the bovine follicular fluid to date [26]. Our results obtained by EIA are comparable to their findings, obtained through high-performance liquid chromatography-tandem mass spectrometry for Aldo, but ten-fold lower for DOC. In the present study, we could not collect blood samples matching the follicular samples due to sampling limitations at the slaughterhouse. This factor prevented us from comparing the concentrations of mineralocorticoids between the serum and the follicular fluid, as done previously in humans [12]. Nevertheless, the follicular fluid concentrations of DOC and Aldo in the present study are somewhat lower or comparable to serum concentrations reported for DOC in pregnant heifers (1–70 ng/ml) [27], and for Aldo in lactating cows and heifers (approximately 100 pg/ml) [28]. Altogether, these results suggest that DOC and Aldo in the bovine follicular fluid are mainly, if not completely, derived from the circulation. However, the appreciable levels of CYP21A2 expression observed in this study, and activity of CYP11B1 reported by Amweg et al. (2017), indicate possible local synthesis of mineralocorticoids in the bovine follicle [11]. In macaques, the concentrations of DOC in the follicular fluid as well as the synthesis of DOC by cultured GC sharply increased after hCG treatment [10]. Likewise, the concentrations of Aldo in the follicular fluid were reported to be much higher than those in the plasma of women who received an ovulatory hCG stimulus [12]. As all our samples were collected before the LH-surge, we may have missed the increase in mineralocorticoid synthesis in the POF triggered by the LH-surge. A further study is necessary to elucidate this possibility.
As CYP21A2 and CYP11B1 also mediate the production of cortisol, we measured the concentrations of cortisol in the paired follicles. Unlike mineralocorticoids, the cortisol level was significantly lower in the atretic follicles compared to that in the healthy follicles. This difference in cortisol levels appeared to be caused by the increased expression of HSD11B2, a dehydrogenase that converts cortisol into inert cortisone, in GC and TL of atretic follicles [18, present study] rather than by changes in the expression levels of CYP21A2 and CYP11B1. The highly significant negative correlation observed in this study between the concentration of cortisol and the expression of HSD11B2 supports this hypothesis.
The presence of mineralocorticoids and the expression of NR3C2 in the bovine follicle indicate that mineralocorticoids may act as a local regulator of follicular functions. As mentioned above, hCG was shown to stimulate DOC production in cultured macaque GC [10]. In that study, P4 production was also stimulated by hCG, and this effect was almost completely negated by the concomitant treatment with spironolactone, an NR3C2 antagonist [10]. These results indicate that the locally produced DOC acts through NR3C2, as an autocrine factor, to stimulate P4 production.
Sneeringer et al. (2011) also suggested that in humans, an increase in the follicular Aldo before ovulation might stimulate oocyte maturation through NR3C2 expressed by the oocyte [12]. The bovine oocyte also expresses NR3C2 [16] as well as HSD11B2, the dehydrogenase that protects NR3C2 from non-specific activation by cortisol [29]. Co-expression of these genes is a typical feature of mineralocorticoid target cells. Thus, we tested this possibility by adding Aldo to the bovine in vitro maturation medium. Aldo enhanced oocyte maturation, and this effect was reversed by eplerenone, a specific NR3C2 antagonist [17]. These results imply that Aldo, as an endocrine, and possibly as a paracrine/autocrine factor, plays an important role in the bovine follicle destined for ovulation.
This study also demonstrated that the expression of CYP21A2 and NR3C2 was much higher in TL than in GC. This notion indicates that the synthesis and action of mineralocorticoids may occur at the outer layer of follicles where the vascular network resides. The bovine theca cells have been shown to express two types of receptors for angiotensin II (Ang II) [30], a vital factor of the renin-angiotensin system, which is a well-known regulatory system of Aldo synthesis in the adrenal gland. For example, Ang II, acting through Ang II receptor type 1, increases the expression of CYP11B2, and thereby stimulates Aldo production in an adrenocortical cell line [31]. The expression of Ang II receptor type 2 was also increased by the GnRH treatment of the bovine theca cells in the preovulatory follicles [32]. Likewise, an ovulatory dose of gonadotropin has been shown to increase the levels of Ang II in the follicular fluid [33]. These findings parallel the gonadotropin-induced increase in follicular mineralocorticoids and CYP21A2 expression observed in primates [10, 12, 13]. In summary, the expression of CYP21A2 and NR3C2 in the presence of renin-angiotensin system in the bovine follicle suggests a localized regulation system for the synthesis of mineralocorticoids.
The CL is renowned for producing P4 in a developmentally regulated fashion, with the highest production at stage III (fully developed CL), followed by a rapid reduction at stage IV (regressing CL). In the present study, the expression of CYP21A2 and NR3C2 paralleled the P4 production pattern, whereas CYP11B1 was only temporarily expressed at stages II and III. These results suggest developmentally regulated synthesis and action of mineralocorticoids in the bovine CL. As mentioned above, an ovulatory stimulus; for example, gonadotropin surge or equivalent hCG treatment, appears to induce mineralocorticoid synthesis in the follicle. In this regard, increases in the expression of mineralocorticoid biosynthetic enzymes along with the development of CL might be triggered by the LH-surge and maintained by the pulsatile secretion of LH thereafter. If this is the case, locally synthesized mineralocorticoid may play an essential role in the luteinizing process, such as increasing P4 synthesis, as reported in the cultured macaque GC [10].
In conclusion, bovine follicles contain appreciable levels of mineralocorticoids, irrespective of their size and physiological status. Although they are likely to be derived from circulation, the expression of CYP21A2 and CYP11B1 in the follicle implies possible de novo synthesis of mineralocorticoids. The expression of NR3C2 in the follicle also indicates local action of mineralocorticoids. On the other hand, the expression of CYP21A2, CYP11B1, and NR3C2 appear to be developmentally regulated in the bovine CL. Collectively, the present results suggest that the bovine ovary is an extra-adrenal organ for the synthesis of mineralocorticoids and a viable target for action.
Acknowledgments
We are grateful to the staff of the Animal Biotechnology Center, Livestock Improvement Association of Japan Inc. for providing bovine ovaries. This work was supported by Grant-in-Aid for Scientific Research (25450460 and 19K06365) to MT from the Japan Society for the Promotion of Science.
References
- 1.Ogishima T, Shibata H, Shimada H, Mitani F, Suzuki H, Saruta T, Ishimura Y. Aldosterone synthase cytochrome P-450 expressed in the adrenals of patients with primary aldosteronism. J Biol Chem 1991; 266: 10731–10734. [PubMed] [Google Scholar]
- 2.Curnow KM, Tusie-Luna M-T, Pascoe L, Natarajan R, Gu J-L, Nadler JL, White PC. The product of the CYP11B2 gene is required for aldosterone biosynthesis in the human adrenal cortex. Mol Endocrinol 1991; 5: 1513–1522. [DOI] [PubMed] [Google Scholar]
- 3.Bülow HE, Bernhardt R. Analyses of the CYP11B gene family in the guinea pig suggest the existence of a primordial CYP11B gene with aldosterone synthase activity. Eur J Biochem 2002; 269: 3838–3846. [DOI] [PubMed] [Google Scholar]
- 4.Bülow HE, Möbius K, Bähr V, Bernhardt R. Molecular cloning and functional expression of the cytochrome P450 11B-hydroxylase of the guinea pig. Biochem Biophys Res Commun 1996; 221: 304–312. [DOI] [PubMed] [Google Scholar]
- 5.Domalik LJ, Chaplin DD, Kirkman MS, Wu RC, Liu WW, Howard TA, Seldin MF, Parker KL. Different isozymes of mouse 11 beta-hydroxylase produce mineralocorticoids and glucocorticoids. Mol Endocrinol 1991; 5: 1853–1861. [DOI] [PubMed] [Google Scholar]
- 6.Ohnishi T, Wada A, Nonaka Y, Sugiyama T, Yamano T, Okamoto M. Effect of calmodulin on aldosterone synthesis by a cytochrome P-450 11β-reconstituted system from bovine adrenocortical mitochondria. J Biochem 1986; 100: 1065–1076. [DOI] [PubMed] [Google Scholar]
- 7.Yanagibashi K, Haniu M, Shively JE, Shen WH, Hall P. The synthesis of aldosterone by the adrenal cortex. Two zones (fasciculata and glomerulosa) possess one enzyme for 11 beta-, 18-hydroxylation, and aldehyde synthesis. J Biol Chem 1986; 261: 3556–3562. [PubMed] [Google Scholar]
- 8.Boon WC, Roche PJ, Hammond VE, Jeyaseelan K, Crawford RJ, Coghlan JP. Cloning and expression analysis of a cytochrome P-450(11 beta) cDNA in sheep. Biochim Biophys Acta 1995; 1260: 109–112. [DOI] [PubMed] [Google Scholar]
- 9.Taves MD, Gomez-Sanchez CE, Soma KK. Extra-adrenal glucocorticoids and mineralocorticoids: evidence for local synthesis, regulation, and function. Am J Physiol Endocrinol Metab 2011; 301: E11–E24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fru KN, VandeVoort CA, Chaffin CL. Mineralocorticoid synthesis during the periovulatory interval in macaques. Biol Reprod 2006; 75: 568–574. [DOI] [PubMed] [Google Scholar]
- 11.Amweg AN, Rodríguez FM, Huber E, Marelli BE, Gareis NC, Belotti EM, Rey F, Salvetti NR, Ortega HH. Detection and activity of 11 beta hydroxylase (CYP11B1) in the bovine ovary. Reproduction 2017; 153: 433–441. [DOI] [PubMed] [Google Scholar]
- 12.Sneeringer R, Penzias AS, Barrett B, Usheva A. High levels of mineralocorticoids in preovulatory follicular fluid could contribute to oocyte development. Fertil Steril 2011; 95: 182–187. [DOI] [PubMed] [Google Scholar]
- 13.Amin M, Simerman A, Cho M, Singh P, Briton-Jones C, Hill D, Grogan T, Elashoff D, Clarke NJ, Chazenbalk GD, Dumesic DA. 21-Hydroxylase-derived steroids in follicles of nonobese women undergoing ovarian stimulation for in vitro fertilization (IVF) positively correlate with lipid content of luteinized granulosa cells (LGCs) as a source of cholesterol for steroid synthesis. J Clin Endocrinol Metab 2014; 99: 1299–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomez-Sanchez EP, Gomez-Sanchez MT, de Rodriguez AF, Romero DG, Warden MP, Plonczynski MW, Gomez-Sanchez CE. Immunohistochemical demonstration of the mineralocorticoid receptor, 11beta-hydroxysteroid dehydrogenase-1 and -2, and hexose-6-phosphate dehydrogenase in rat ovary. J Histochem Cytochem 2009; 57: 633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tetsuka M, Milne M, Simpson GE, Hillier SG. Expression of 11β-hydroxysteroid dehydrogenase, glucocorticoid receptor, and mineralocorticoid receptor genes in rat ovary. Biol Reprod 1999; 60: 330–335. [DOI] [PubMed] [Google Scholar]
- 16.Robert C, Barnes FL, Hue I, Sirard M-A. Subtractive hybridization used to identify mRNA associated with the maturation of bovine oocytes. Mol Reprod Dev 2000; 57: 167–175. [DOI] [PubMed] [Google Scholar]
- 17.Tetsuka M, Kashima A. Mineralocorticoid modulates bovine oocyte nuclear maturation through mineralocorticoid receptor. In: Program of the 4th World Congress of Reproductive Biology; 2017; Okinawa, Japan. Abstract P1-47.
- 18.Tetsuka M, Nishimoto H, Miyamoto A, Okuda K, Hamano S. Gene expression of 11β-HSD and glucocorticoid receptor in the bovine (Bos taurus) follicle during follicular maturation and atresia: the role of follicular stimulating hormone. J Reprod Dev 2010; 56: 616–622. [DOI] [PubMed] [Google Scholar]
- 19.Ireland JJ, Murphee RL, Coulson PB. Accuracy of predicting stages of bovine estrous cycle by gross appearance of the corpus luteum. J Dairy Sci 1980; 63: 155–160. [DOI] [PubMed] [Google Scholar]
- 20.Murasawa M, Takahashi T, Nishimoto H, Yamamoto S, Hamano S, Tetsuka M. Relationship between ovarian weight and follicular population in heifers. J Reprod Dev 2005; 51: 689–693. [DOI] [PubMed] [Google Scholar]
- 21.Nishimoto H, Hamano S, Hill GA, Miyamoto A, Tetsuka M. Classification of bovine follicles based on the concentrations of steroids, glucose and lactate in follicular fluid and the status of accompanying follicles. J Reprod Dev 2009; 55: 219–224. [DOI] [PubMed] [Google Scholar]
- 22.Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics 2012; 13: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bijault C, Dehennin L. Steroid 21-hydroxylase activity in equine ovarian follicles evidenced by isotope dilution-mass spectrometry. J Steroid Biochem Mol Biol 1991; 38: 165–172. [DOI] [PubMed] [Google Scholar]
- 24.Yazawa T, Uesaka M, Inaoka Y, Mizutani T, Sekiguchi T, Kajitani T, Kitano T, Umezawa A, Miyamoto K. Cyp11b1 is induced in the murine gonad by luteinizing hormone/human chorionic gonadotropin and involved in the production of 11-ketotestosterone, a major fish androgen: conservation and evolution of the androgen metabolic pathway. Endocrinology 2008; 149: 1786–1792. [DOI] [PubMed] [Google Scholar]
- 25.Amweg AN, Paredes A, Salvetti NR, Lara HE, Ortega HH. Expression of melanocortin receptors mRNA, and direct effects of ACTH on steroid secretion in the bovine ovary. Theriogenology 2011; 75: 628–637. [DOI] [PubMed] [Google Scholar]
- 26.Summers AF, Pohlmeier WE, Sargent KM, Cole BD, Vinton RJ, Kurz SG, McFee RM, Cushman RA, Cupp AS, Wood JR. Altered theca and cumulus oocyte complex gene expression, follicular arrest and reduced fertility in cows with dominant follicle follicular fluid androgen excess. PLoS One 2014; 9: e110683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wise TH, Sorensen AM, Jr, Fleeger JL. Quantitation of deoxycorticosterone and its relationship to progesterone in the prepartum bovine. Steroids 1975; 26: 17–28. [DOI] [PubMed] [Google Scholar]
- 28.Roussel JD, Clement TJ, Aranas TJ. Changes of aldosterone in blood serum of dairy cattle during estrous cycle. J Dairy Sci 1983; 66: 1734–1737. [DOI] [PubMed] [Google Scholar]
- 29.Tetsuka M, Takagi R, Ambo N, Myat TS, Zempo Y, Onuma A. Glucocorticoid metabolism in the bovine cumulus-oocyte complex matured in vitro. Reproduction 2016; 151: 73–82. [DOI] [PubMed] [Google Scholar]
- 30.Schauser KH, Nielsen AH, Winther H, Dantzer V, Poulsen K. Localization of the renin-angiotensin system in the bovine ovary: cyclic variation of the angiotensin II receptor expression. Biol Reprod 2001; 65: 1672–1680. [DOI] [PubMed] [Google Scholar]
- 31.Nogueira EF, Xing Y, Morris CAV, Rainey WE. Role of angiotensin II-induced rapid response genes in the regulation of enzymes needed for aldosterone synthesis. J Mol Endocrinol 2009; 42: 319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonçalves PB, Ferreira R, Gasperin B, Oliveira JF. Role of angiotensin in ovarian follicular development and ovulation in mammals: a review of recent advances. Reproduction 2012; 143: 11–20. [DOI] [PubMed] [Google Scholar]
- 33.Acosta TJ, Ozawa T, Kobayashi S, Hayashi K, Ohtani M, Kraetzl WD, Sato K, Schams D, Miyamoto A. Periovulatory changes in the local release of vasoactive peptides, prostaglandin f(2α), and steroid hormones from bovine mature follicles in vivo. Biol Reprod 2000; 63: 1253–1261. [DOI] [PubMed] [Google Scholar]