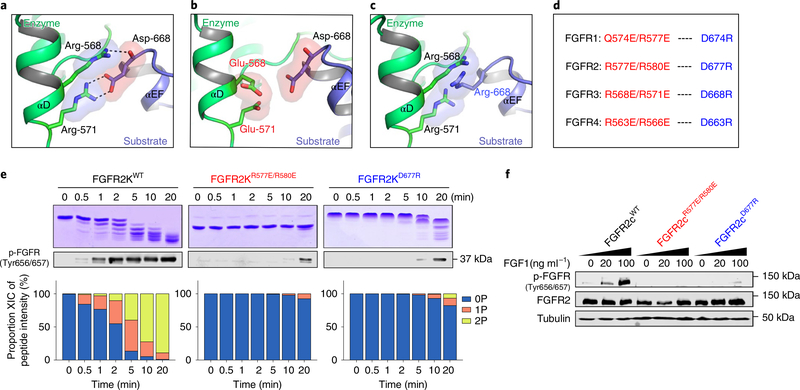
Fig. 4 |. Functional validation of the crystallographically deduced A-loop-transphosphorylating mechanism in vitro and in vivo.
a, Expanded view of the FGFR3KR669E asymmetric complex interface highlighting the key contribution of (i) the salt bridge between R571 (enzyme) and D668 (substrate), and (ii) the hydrogen bond between R568 (enzyme) and D668 (substrate) backbone (in each case shown as dashed lines). b,c, Introduction of a R568E/R571E double substitution in the enzyme kinase (b) or a D668R single substitution in the substrate kinase (c) are predicted to inhibit A-loop-transphosphorylating asymmetric complex formation by eliminating both salt-bridge and hydrogen-bonding interactions and by introducing electrostatic clashes. d, Equivalent residues in FGFR1–FGFR4 that mediate salt bridges and hydrogen bonds at the asymmetric complex interface and their corresponding substitution to residues with opposite charge, engineered to abolish dimerization. e, Kinetic analyses by native gel electrophoresis (top), immunoblotting (middle) and time-resolved LC–MS of A-loop-tyrosine phosphorylation (bottom) in wild-type FGFR2Ks and its variants harboring mutations predicted to disrupt the asymmetric complex. Kinase assays were done independently twice with similar results. f, Immunoblot analyses of whole lysates of buffer-treated or FGF1-stimulated L6 myoblasts overexpressing either full-length wild-type FGFR2 or corresponding variants harboring dimer-breaking substitutions. Blots were probed with anti-p-FGFR (Y656/Y657), anti-FGFR2 and anti-β-tubulin antibodies. Experiments were performed in biological triplicates with similar results. Full-length gels are shown in Supplementary Fig. 15b,c.