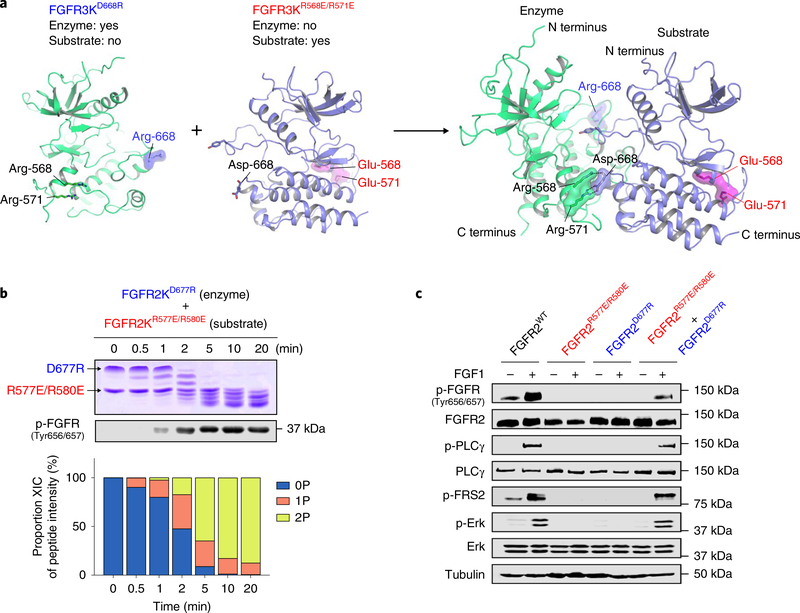
Fig. 5 |. In vitro and in vivo complementation assays reinforce the existence of an asymmetric A-loop-tyrosine transphosphorylation complex.
a, Cartoon representation of heterodimerization of FGFR3D668R and FGFR3R568E/R571E in which FGFR3D668R assumes the role of enzyme, while FGFR3R568E/R571E acts as substrate. Locations of mutated residues (E568 and E571, red; R668, blue) are highlighted. b, Kinetic analysis of phosphorylation of A-loop tyrosines in reactions containing equimolar amounts of FGFR2KD677R and FGFR2KR577E/R580E by native gel electrophoresis (top), immunoblotting with an anti-p-FGFR antibody (middle) and time-resolved LC–MS (bottom). Kinase assays were done independently twice with similar results. c, Lysates from buffer-treated or FGF1-treated L6 myoblasts stably expressing either wild-type FGFR2c, FGFR2cR577E/R580E or FGFR2cD677R alone, or co-expressing FGFR2cR577E/R580E and FGFR2cD677R, in each case analyzed by immunoblotting using antibodies specific for selected target proteins and their phosphorylated forms. An anti-β-tubulin antibody was used as a loading control. Experiments were performed in biological triplicates with similar results. Full-length gels are shown in Supplementary Fig. 15d,e.