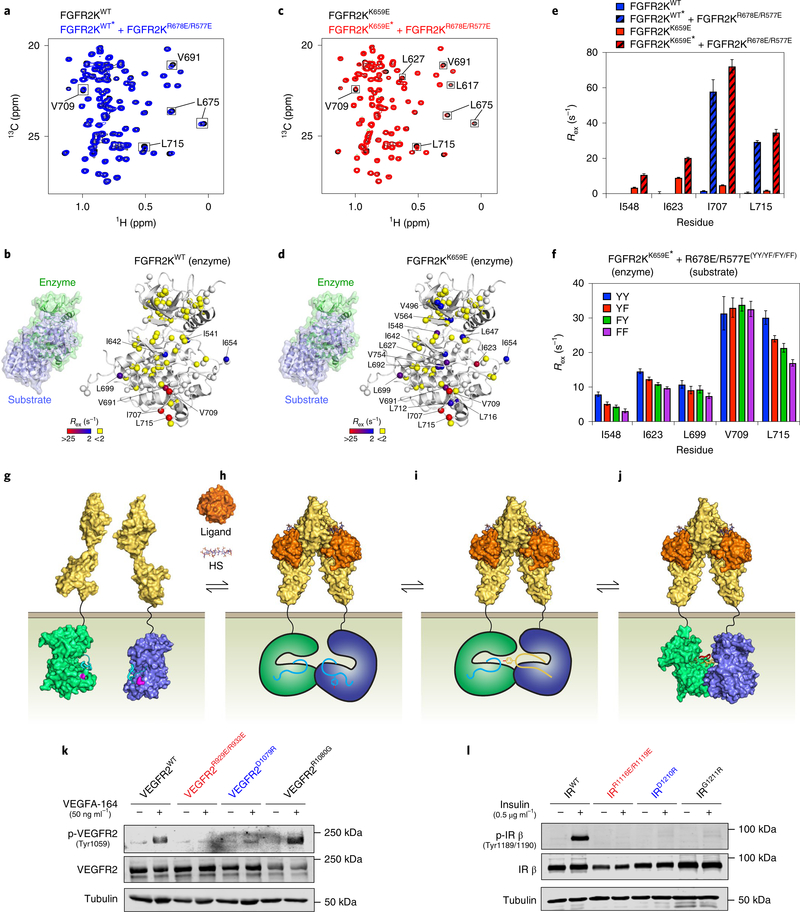
Fig. 6 |. Asymmetric complex formation induces reciprocal allosteric changes in enzyme and substrate kinases.
a,c, Overlays of 1H/13C HMQC (leucine/valine region) spectra of 0.4 mM isotopically labeled FGFR2KWT (a; blue) or FGFR2KK659E (c; red) either alone or together with 0.8 mM unlabeled substrate kinase (that is, FGFR2KR577E/R678E). Peaks sustaining >20% loss of intensity are boxed. Experiments were performed independently twice with similar results. b,d, Rex values (with range depicted by a boxed colored bar) derived from CPMG relaxation dispersion experiments for FGFR2KWT (b) or FGFR2KK659E (d) mixed with unlabeled FGFR2KR577E/R678E mapped onto the enzyme-acting kinase in the asymmetric complex crystal structure. e, Changes in Rex values of selected residues in FGFR2KWT or FGFR2KK659E enzyme kinase induced upon addition of substrate (that is, FGFR2KR577E/R678E). f, Reductions in CPMG-derived Rex values in FGFR2KK659E enzyme kinase when A-loop tyrosines (annotated YY) of FGFR2KR577E/R678E substrate kinase are substituted to YF, FY and FF. e,f, n = 1 using independent samples for each set of CPMG measurements acquired at two magnetic field strengths; error bars reflect the fitted error to equation (2). a,c,e,f, Isotopically enriched kinases contained in mixtures are indicated by asterisks. g–j, Induced-fit model for A-loop-tyrosine transphosphorylation. g, Asymmetric complex formation of FGFR kinases (enzyme and substrate in green and blue, respectively) is thermodynamically inhibited by a charge repulsion between K659 in the enzyme-acting kinase and R669 in the incoming substrate-acting kinase (both residues highlighted in pink). h, Energetic gains in extracellular FGF-induced FGFR dimerization offset these repulsive forces, facilitating formation of a C lobe–C lobe-mediated asymmetric kinase dimer. HS, heparan sulfate. i, Asymmetric complex formation imparts upon the substrate A-loop a more phosphorylatable conformation (indicated as a change in color to yellow). j, This encourages the A-loop of the enzyme to adopt the active state (depicted by a change in color to red), resulting in the formation of an A-loop-tyrosine transphosphorylation complex as revealed by the crystal structure. k,l, Immunoblot analyses of L6 myoblast cell lines overexpressing either full-length mouse wild-type VEGFR2 (k) or human wild-type insulin receptor (IR) (l), together with variants harboring either a R1080G substitution (k) or a G1211R substitution (l) (in each case corresponding to FGFR2 R678) plus dimer-disrupting substitutions R929E/R932E and D1079R (k) or R1116E/R1119E and D1210R substitutions (l) (in each case corresponding to FGFR2 R577E/R580E and D677R). Cells were stimulated with either VEGF (k) or insulin (l) at the concentrations shown. Whole-cell lysates were analyzed by immunoblotting using antibodies specific for p-VEGFR2, VEGFR2 (k) or antibodies specific for phosphorylated human insulin receptor (p-hIR) or human insulin receptor (hIR) (l). k,l, An antibody to β-tubulin was used as a loading control. Experiments were performed in biological triplicates with similar results. Full-length gels are shown in Supplementary Fig. 15f,g.