Figure 1.
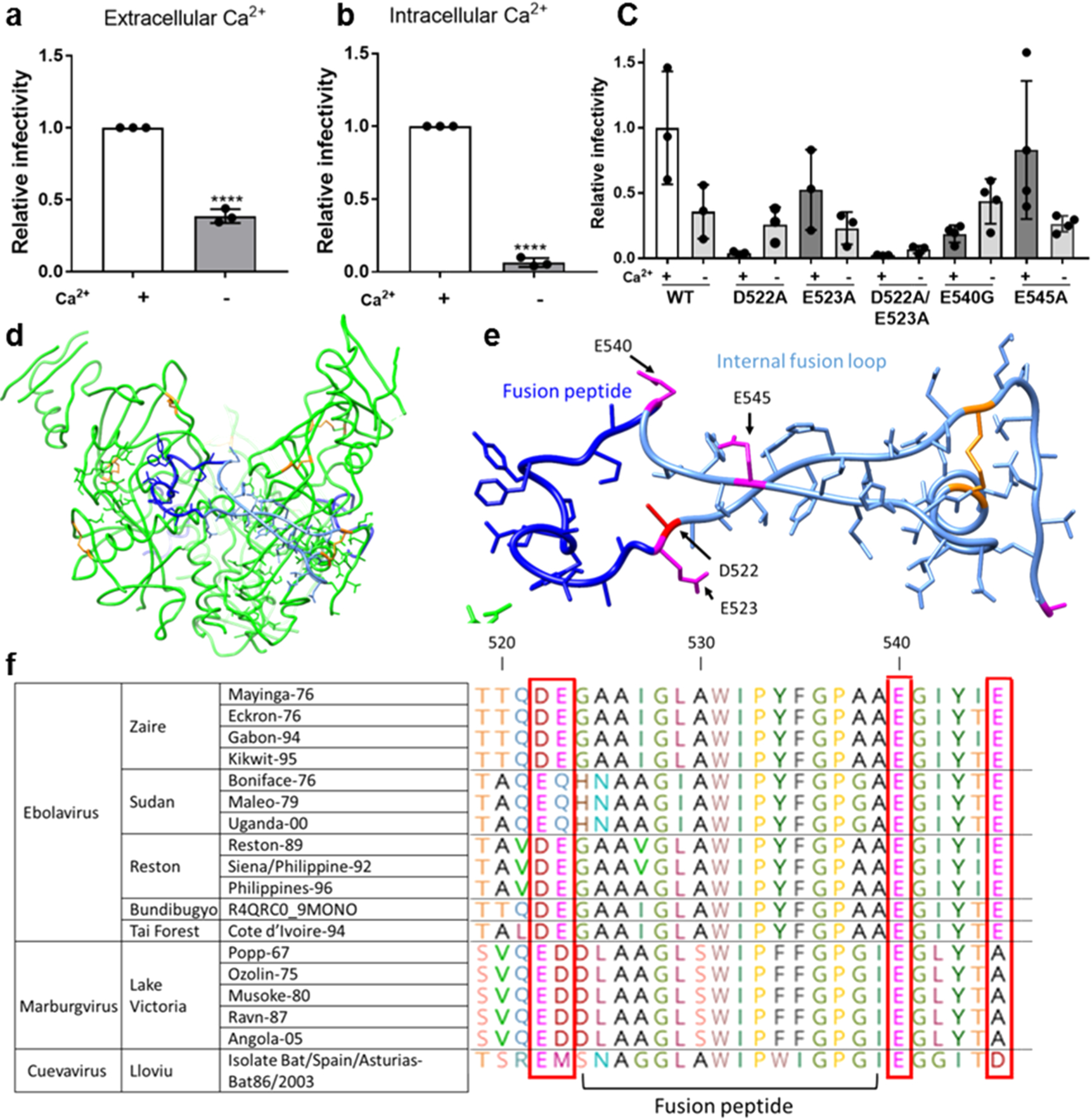
Ebolavirus entry is enhanced in the presence of extracellular and intracellular calcium. Viral particles pseudotyped with Zaire ebolavirus GP were added to (a) Vero E6 cells and allowed to internalize in calcium-free DMEM (−Ca2+) or DMEM with 1.8 mM calcium (+Ca2+) for 2 h to assess the impact of extracellular calcium and (b) Vero E6 cells pretreated with 50 μM of the chelator BAPTA AM or DMSO for 1 h. Pseudovirus particles were then added to cells with 50 μM BAPTA AM or DMSO and allowed to internalize for 2 h. Data was normalized so that infection +Ca2+ was equal to 1. Error bars represent the standard deviation for 3 independent experiments. (c) Infectivity of pseudotyped viral particles with WT or mutated EBOV GP. Data was normalized so that infectivity of WT EBOV in the presence of Ca2+ was 1. Error bars represent s.d. from 3 independent experiments, each with 3 technical replicates, with the exception of E545A and E54G, for which 4 independent experiments were conducted. Dots represent the mean of the technical replicates. p values were determined by the one-way ANOVA comparison to WT. (d) Trimer of Zaire EBOV GP with fusion peptide (dark blue), fusion loop (pale blue), and disulfide bonds (orange). (e) Zaire EBOV fusion loop and nearby negatively charged residues that may be involved in binding calcium. (f) Glycoprotein sequences of different filovirus strains were retrieved from UNIPROT and aligned using CLUSTAL. Horizontal lines demarcate different species.
