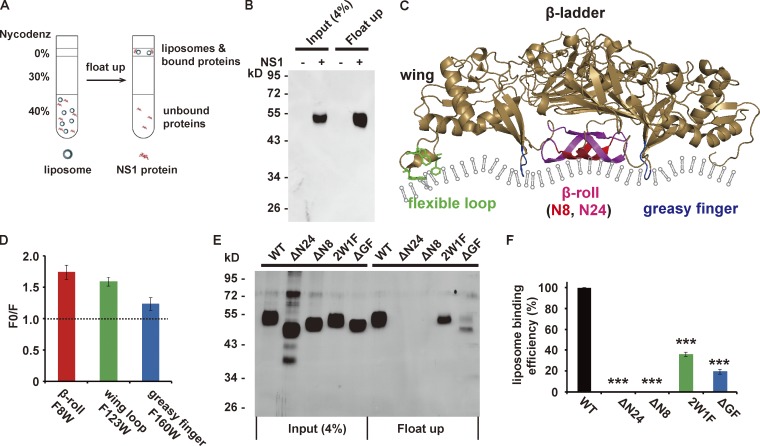
Figure 2.
ZIKV NS1 binds to the membrane via hydrophobic insertion. (A) Schematic diagram of the liposome cofloating assay. The ZIKV NS1 protein was incubated with liposomes (∼100 nm diameter) for 4 h at 37°C, and the protein–liposome mixture was then ultracentrifuged against 40%, 30%, and 0% Nycodenz gradients. Membrane-bound NS1 cofloated with liposomes, whereas unbound NS1 remained on the bottom. (B) NS1 cofloats with liposomes. Input and cofloating samples of NS1 were analyzed by silver staining. (C) Structure of the ZIKV NS1 dimer (PDB 5k6k). The hydrophobic membrane-binding regions are marked in different colors: red (eight N-terminal residues of the β-roll), red plus pink (24 N-terminal residues of the β-roll), green (wing flexible loop; three hydrophobic residues, W115, W118, and F123, are shown as sticks), and blue (GF). (D) Doxyl quenching of Trp in peptides derived from NS1 hydrophobic regions. Peptides with a single Trp were mixed with 12-doxyl-labeled (F) or unlabeled (F0) liposomes, and the fluorescence of Trp was monitored at 330 nm. The quenching level is presented as F0/F. (E and F) Liposome-binding activity of WT and mutated NS1. (E) Silver-stained gel for input and cofloated NS1. (F) Quantification of the cofloating assay. Liposome-binding efficiency was defined as the percentage of cofloated NS1 to the input, and the binding efficiency of WT NS1 was set to 100%. The data are the mean ± SEM. The P values are obtained from a two-tailed t test. ***, P < 0.001.