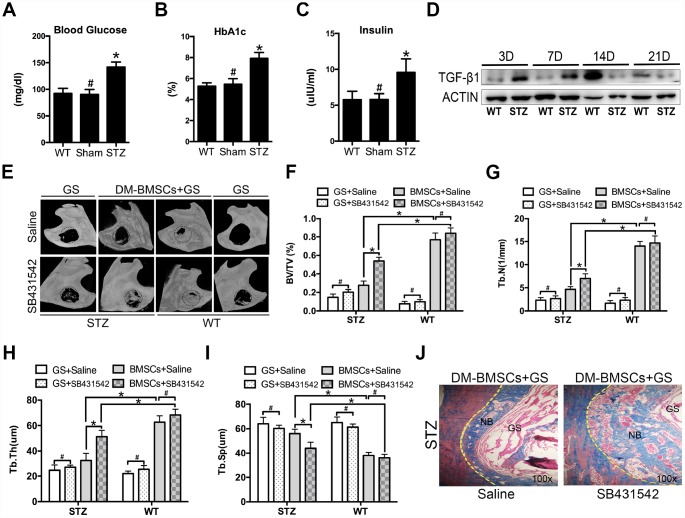
Figure 5.
Suppression of TGF-β1 signaling promotes repair of mandible defects in diabetic rat. Blood was collected from the tail veins after overnight fasting, and blood glucose levels were measured using a glucometer (A). Blood samples were taken from anesthetized rats through retrobulbar venous plexuses. Serum insulin and HBA1c levels were measured in a clinical laboratory (B, C). Full thickness bone defect (1×3mm) was made in the mandibular margins, and calluses were harvested at postoperative 3, 7, 14, and 21 days. The expression of TGF-β1 was measured by western blot (D). Mandibular critical-sized bone defects (diameter 5mm) were made in an STZ-induced diabetic rat and WT rat model, and human DM-BMSCs with gelatin sponge (GS) or GS alone were implanted into defects. SB431542 or saline were injected into the local defect once every 2 days for 1 week. After 8 weeks, micro-CT images were taken (E). Trabecular bone volume fraction (BV/TV), trabecular number (Tb.N.), trabecular thickness (Tb.Th.), trabecular separation (Tb.Sp.) were analyzed (F–I). New bone formation in STZ group implantation of DM-BMSCs with GS were evaluated by Masson trichrome staining (J). Black dotted lines in E indicate the size and location of defect. Yellow dotted lines in J indicate the bonder of defect. NB, new bone, GS, gelation sponge. Data are presented as the mean ± standard deviation, n=3. *p<0.05, #p>0.05.