Abstract
Background
Understanding the interactions between increased insecticide resistance and resting behaviour patterns of malaria mosquitoes is important for planning of adequate vector control. This study was designed to investigate the resting behavior, host preference and rates of Plasmodium falciparum infection in relation to insecticide resistance of malaria vectors in different ecologies of western Kenya.
Methods
Anopheles mosquito collections were carried out during the dry and rainy seasons in Kisian (lowland site) and Bungoma (highland site), both in western Kenya using pyrethrum spray catches (PSC), mechanical aspiration (Prokopack) for indoor collections, clay pots, pit shelter and Prokopack for outdoor collections. WHO tube bioassay was used to determine levels of phenotypic resistance of indoor and outdoor collected mosquitoes to deltamethrin. PCR-based molecular diagnostics were used for mosquito speciation, genotype for knockdown resistance mutations (1014S and 1014F) and to determine specific host blood meal origins. Enzyme-linked Immunosorbent Assay (ELISA) was used to determine mosquito sporozoite infections.
Results
Anopheles gambiae s.l. was the most predominant species (75%, n = 2706) followed by An. funestus s.l. (25%, n = 860). An. gambiae s.s hereafter (An. gambiae) accounted for 91% (95% CI: 89–93) and An. arabiensis 8% (95% CI: 6–9) in Bungoma, while in Kisian, An. arabiensis composition was 60% (95% CI: 55–66) and An. gambiae 39% (95% CI: 34–44). The resting densities of An. gambiae s.l and An. funestus were higher indoors than outdoor in both sites (An. gambiae s.l; F1, 655 = 41.928, p < 0.0001, An. funestus; F1, 655 = 36.555, p < 0.0001). The mortality rate for indoor and outdoor resting An. gambiae s.l F1 progeny was 37% (95% CI: 34–39) vs 67% (95% CI: 62–69) respectively in Bungoma. In Kisian, the mortality rate was 67% (95% CI: 61–73) vs 76% (95% CI: 71–80) respectively. The mortality rate for F1 progeny of An. funestus resting indoors in Bungoma was 32% (95% CI: 28–35). The 1014S mutation was only detected in indoor resitng An. arabiensis. Similarly, the 1014F mutation was present only in indoor resting An. gambiae. The sporozoite rates were highest in An. funestus followed by An. gambiae, and An. arabiensis resting indoors at 11% (34/311), 8% (47/618) and 4% (1/27) respectively in Bungoma. Overall, in Bungoma, the sporozoite rate for indoor resting mosquitoes was 9% (82/956) and 4% (8/190) for outdoors. In Kisian, the sporozoite rate was 1% (1/112) for indoor resting An. gambiae. None of the outdoor collected mosquitoes in Kisian tested positive for sporozoite infections (n = 73).
Conclusion
The study reports high indoor resting densities of An. gambiae and An. funestus, insecticide resistance, and persistence of malaria transmission indoors regardless of the use of long-lasting insecticidal nets (LLINs). These findings underline the difficulties of controlling malaria vectors resting and biting indoors using the current interventions. Supplemental vector control tools and implementation of sustainable insecticide resistance management strategies are needed in western Kenya.
Background
Malaria still remains a major public health concern in sub-Saharan Africa, responsible for an estimated 228 million cases and 405,000 deaths despite the massive investments in scaling-up indoor anti-vector interventions [1]. Remarkable advances in the fight against malaria have been achieved within the past decade mainly through the massive scale-up of long-lasting insecticide-treated nets (LLINs) and indoor residual spraying (IRS) in many localities [2,3]. Despite the increased efforts, it is worrying that no significant progress has been made in reducing global malaria cases in the year 2015–2017 period [4], with some regions in sub-Saharan Africa, previously reported to experience a resurgence of malaria including western Kenya [5]. This transmission recurrence is partly attributed to the emergence of insecticide resistance and behavioural modification that have arisen as an adaptation by mosquitoes in response to high use of insecticides for vector control [6,7]. All these factors have the potential to weaken malaria control programs thus posing a serious threat in the fight against malaria [8].
The current vector control interventions take advantage of susceptible mosquito behaviors. These interventions are based on the observation that malaria vectors prefer to bite humans indoors late at night and often rest inside houses after blood feeding hence, they will be exposed to sufficient levels of insecticides which will either kill them or reduce their longevity thus affecting their vectorial capacity [4]. In sub-Saharan Africa insecticide-treated net (ITN) ownership is estimated to have increased from 3% in 2000 to 83% in the period of 2015–2017[4]. In Kenya, the government rolled out the universal bed net programme where every two persons in a household were provided with a free ITN. The ITN ownership rose from 12.8% in 2004 to over 80% in 2015[9,10] The increased use of indoor interventions may pose stress on the indoor feeding and resting of malaria vectors leading to either behavioural defense [11] or physiological defense [6]. Malaria vectors have been shown to adapt to changing environment due to either behavioural avoidance or selection of mutations and recombination that favour their survival in the presence of insecticides threatening the efficacy of the current indoor-based vector control tools [6,12] and the resulting increase in residual transmission [13]. Insecticide resistance is common in sub-Saharan Africa with some regions reporting resistance to all classes of insecticides [14,15]. In Kenya, the target site and metabolic resistance mechanisms play a major role in pyrethroid resistance [16,17]. The primary malaria vectors in Kenya belong to An. gambiae complex and An. funestus group due to their anthropophilic and endophilic behaviours that makes them be more efficient in malaria transmission [18–20]. With the scale-up of indoor-based vector control tools mosquitoes have changed behaviours; some are biting and resting indoors whilst others have changed to prefer biting and resting outdoors. Behavioral modifications including changes in biting time and location [21–23], changes in host choice and shift from endophilic (i.e. resting in houses) to exophilic (i.e. resting outdoors) behavior have been associated with long-term use of insecticide-based interventions [24,25]. Knowledge of the resting habits of resistant vectors and their feeding preference may predict the intensity of malaria transmission. It is hypothesized that insecticide-resistant malaria vectors could bite and rest indoors in the presence of interventions whilst susceptible ones bite and rest outdoors. Additionally, behaviors of malaria vectors have been shown to differ on small geographical scales, further complicating malaria elimination efforts[26]. Understanding how the resting habits of malaria vectors change in response to current indoor-based vector control interventions is important for sustaining vector control. These behavioral modifications and physiological resistance in most of the malaria vectors have been shown to contribute to maintaining malaria transmission [12,27].
In order to improve vector control intervention strategies, it is crucial to characterize the behavioral patterns of each species of a particular vectorial system in their specific settings over time and in a range of environmental changes, especially with increasing pyrethroid resistance. The objective of this study was to investigate the species diversity of malaria vectors, their resting behavior, and the distribution of infections in two ecological settings of western Kenya with different levels of insecticide resistance. This information could provide a better understanding of the interactions between increased insecticide resistance phenotypes in field malaria vector population and the subsequent resting behaviour patterns in the presence of the current indoor intervention.
Methods
Study site
The study was carried out in Kisian (00.02464°S, 033.60187°E, altitude 1,280–1,330 m above sea level), Kisumu county and Bungoma (00.54057°N, 034.56410°E, altitude 1386–1,545 m above sea level) in Bungoma County, all in western Kenya. Kisian is located in the lowland area around Lake Victoria in western Kenya. An. gambiae sensu stricto (s.s.), An. arabiensis and An. funestus are the main vectors of malaria in this region [17,28]. Bungoma County is located in malaria epidemic-prone highland area. The sites experience a bimodal pattern of rainfall, with the long rainy season (April—July) which triggers peak malaria transmission period and the short rainy season (October-November) and year-to-year variation. The hot and dry season is from January to March which marks the lowest transmission period[5] Both sites are endemic for Plasmodium falciparum malaria. The malaria vector population in both sites include An. gambiae and An. arabiensis and Anopheles funestus [16,17]
Mosquito sampling
Mosquitoes were sampled during the middle of the long dry season (February-March) and four weeks after the start of the long rainy season (May-July) in 2018. Indoor resting malaria vectors were sampled using pyrethrum spray catches (PSCs) in sixty (60) randomly selected houses from 06:00 to 09:00 h [23]. The prokopack aspirator (John W Hock, Gainesville, FL, USA) was used to collect mosquitoes resting indoors and outdoors from the selected houses every morning. For indoor collections, mosquitoes resting on the walls and under the roof of the house or ceiling, under the beds were systematically aspirated. Outdoor sampling points included granaries, kitchens and evening outdoor human resting points. Outdoor resting mosquitoes were additionally collected from pit shelters constructed according to Muirhead-Thomson's method [29] within 20 m of each selected house, resting mosquitoes in the cavities created in the pit shelter were collected from 06:00 to 09:00 h by using Prokopack. Clay-pots were used to collect outdoor resting mosquitoes. The pots were placed then left outdoors from 18:00 to 06:00h at about 5m from the window of selected houses [30]. Resting mosquitoes in the pots were collected in the morning from 06:00 to 09:00h by placing a white mesh from a mosquito cage over the mouth and agitating the mosquitoes inside the pot, causing them to fly and move to the cage [30,31]. The pot was checked for the remaining mosquitoes and were collected using an aspirator to a well-labeled paper cup. Anopheline mosquitoes were sorted morphologically according to the identification keys described by [32]. Female mosquitoes were further classified according to their gonotrophic status. Mosquitoes from each collection method were stored in separately labeled vials and preserved by desiccation.
Some of the collected indoor and outdoor resting mosquitoes that were either blood-fed or gravid from the two sites were kept in paper cups covered with moistened cotton towels and transported to the insectary at Kenya medical research institute in Kisumu. Gravid An. gambiae s.l and An. funestus s.l females were provided with oviposition cups. Eggs laid were allowed to hatch in spring water in small trays and larvae reared on a mixture of tetramin (fish food) and brewer’s yeast provided daily under controlled standard insectary conditions with a temperature range of 26± 2°C and 70% to 80% relative humidity. Emerging adults were provided with a 10% sugar solution until ready to be used for bioassay tests.
WHO resistance bioassays
To assess susceptibility or resistance of F1 progeny of mosquitoes caught from different locations(indoor and outdoor) and study sites, emerging female adults aged 2–5 days were exposed to 0.05% deltamethrin following the standard WHO tube test protocol[33] for 1 h. The knockdown time (KDT) of females was reported every 10 min during the 60 min exposure period. After the 1 h exposure, surviving mosquitoes were transferred to recovery tubes and provided with 10% sucrose for 24 h holding period. Mosquitoes alive 24 h after the 60-min insecticide-exposure time were classified as resistant. Mortality was scored after the 24 h recovery period.
Anopheline species discrimination
Sibling species of the An. gambiae and An. funestus complexes were distinguished using conventional PCR [34,35]. DNA was extracted from mosquito legs and wings using ethanol precipitation method [36].
Detection of sporozoite infectivity
The head and thorax of individual mosquitoes samples collected were used to detect the presence of P. falciparum sporozoites using enzyme-linked immunosorbent assays (ELISA) method as described by Wirtz et al. [37].
Detection of blood meal sources using polymerase chain reaction (PCR)
The abdominal section of blood-fed Anopheles mosquitoes were cut transversely between the thorax and the abdomen. Genomic DNA was extracted from mosquito abdomens using ethanol precipitation method as described by Collins et al. [36]. One universal reverse primer and five animal-specific forward primers (human, cow, goat, pig, and dog) were used for amplification of cytochrome b gene, encoded in the mitochondrial genome to test for specific host blood meal origins using conventional PCR [38].
Genotyping for kdr mutations
DNA was extracted from adult An. gambiae and An. arabiensis mosquitoes as earlier described [34]. Real-time (RT) PCR was used to detect mutations at amino acid position 1014 of the voltage-gated sodium channel (Vgsc) using a modification of the protocol by Bass et al. [39]. Samples were genotyped for both Vgsc-1014S and 1014F kdr alleles.
Scientific and ethical clearance
The institutional review board of Kenya Medical Research Institute granted ethical review and approval (Ref: KEMRI/SERU/CGHR/085/3434). Prior to the commencement of data collection, a detailed explanation of the aims, study procedures, risks and benefits were provided to community leaders and participants of each study site. Informed consent was obtained from the household heads. Participation was voluntary and household heads were free to withdraw from the study in case of any inconvenience.
Data analysis
Resting density of Anopheline mosquitoes was calculated as the number of female mosquitoes per trap/night for each trapping method. Analysis of variance (ANOVA) was used to compare malaria vector density between indoor and outdoor locations. Chi-square was used to test the difference in seasonal abundance and malaria vector species composition between resting locations (indoor and outdoor). Human blood index (HBI) was calculated as the proportion of blood-fed mosquito samples that had fed on humans to the total tested for blood meal origins [40]. Bovine, goat, dog, and pig blood indices were also calculated in a similar way. Mixed blood meals were included in the calculation of blood meal indices [41].
The sporozoite infection rate (IR) expressed as the proportion of mosquitoes positive for Plasmodium sporozoite was calculated by dividing the number of sporozoite positive mosquitoes by the total number of mosquitoes assayed. The frequency of the resistance allele was calculated using the Hardy-Weinberg equilibrium test for kdr genotypes. Data were analyzed using R software packages.
Results
Indoor and outdoor Anopheline mosquito composition
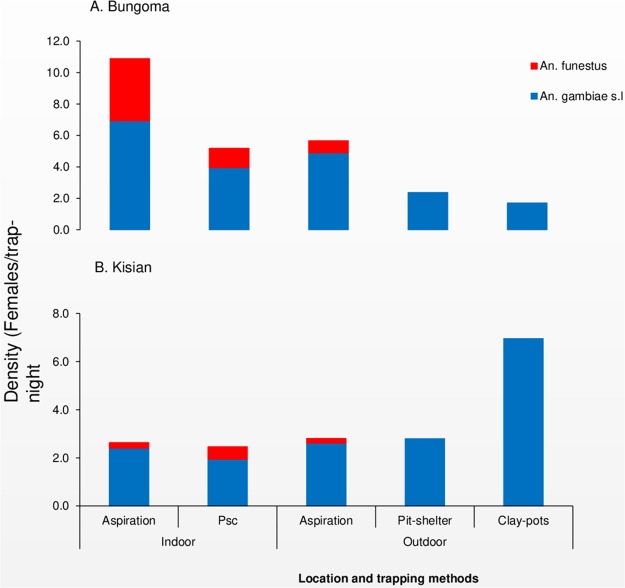
A total of 2,706 and 860 female Anopheline mosquitoes were collected from Bungoma (highland site) and Kisian (lowland site) respectively during the study period. Anopheles gambiae s.l was the most abundant species accounting for 70% (95% CI: 68–71) in Bungoma and 91% (95% CI: 89–93) in Kisian followed by An. funestus 31% (95% CI: 29–32) and 10% (95% CI: 7–11) respectively. In Bungoma out of 1880 An. gambiae s.l collected, 85% (1606/1880) was caught resting indoors and 15% (274/1880) were caught resting outdoors. For An. funestus, 97% (798/826) were caught resting indoors and 3% (28/826) were caught outdoors. In Kisian, 58% (453/781) An. gambiae s.l were resting indoors and 42% (328/781) were caught resting outdoors. For An. funestus, 91% (72/79) were collected indoors and 9% (7/79) was caught resting outdoors (S1 Table). The mean indoor resting density of An. gambiae s.l from both sites was significantly higher than outdoor resting density (F1, 655 = 41.928, p < 0.0001). The mean indoor resting density for An. funestus was also higher than outdoor resting density (F1, 655 = 36.555, p < 0.0001) (Fig 1).
Fig 1. Indoor and outdoor resting density of female Anopheles mosquitoes collected per trapping method A: Highland site (Bungoma) and B: Lowland site (Kisian), western Kenya.
Anopheles gambiae and An. funestus sibling species composition
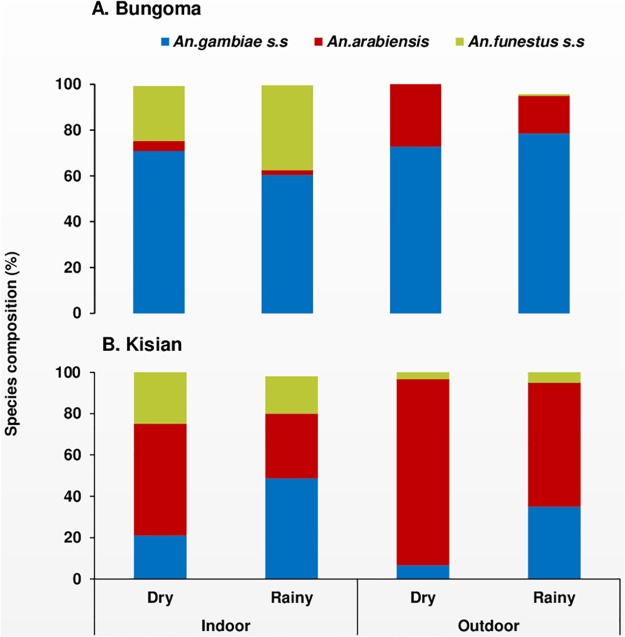
For species identification, a sub-sample of 1,566 from both sites (1,172 An. gambiae s.l and 394 An. funestus s.l) were used to discriminate the sibling species. In Bungoma, 843 samples of An. gambiae s.l were analysed. Of these 91% (95% CI: 89–93) were An. gambiae and 8% (95% CI: 6–9) were An. arabiensis. Overall, of the three vector species, An. gambiae (66%, 95% CI: 64–70) was the predominant malaria vector in Bungoma followed by An. funestus (28%, 95% CI: 25–30) and An. arabiensis (5%, 95% CI: 4–7). There was a significant difference between indoor and outdoor locations in terms of mosquito species composition in Bungoma (X2 = 122.96, df = 2, p < 0.0001). In Kisian, out of the 329 samples analysed 60% (95% CI: 55–66) were An. arabiensis and 39% (95% CI: 34–44) were An. gambiae. Overall An. arabiensis (49%, 95% CI: 44–54) was the most abundant vector species followed by An. gambiae (32%, 95% CI: 27–36) and An. funestus (19%, 95% CI: 15–23, Fig 2). All the An. funestus s.l assayed from the two study sites were all An. funestus s.s (hereafter An. funestus).
Fig 2. Seasonal composition and Anopheles sibling species composition resting indoors and outdoors in A: Highland site (Bungoma) and B: Lowland site (Kisian), western Kenya.
In Bungoma, the seasonal composition of An. gambiae and An. funestus species was higher during the rainy season (57%, 95% CI: 53–61) and (67%, 95% CI: 63–73) compared to the dry season (43%, 95% CI: 39–47) and vs (32%, 95% CI: 25–37)(X2 = 16.28, df = 2, p < 0.0003) respectively. In contrast, in Kisian the overall seasonal prevalence of the three vector species composition was higher during the dry season than rainy season (An. arabiensis, 68% (95% CI: 61–76) and 32% (95% CI: 24–40), An. funestus, 63 (95% CI: 52–74) and 37% (95% CI: 21–42); X2 = 30.42, df = 2, p < 0.0001, Fig 2).
Phenotypic resistance
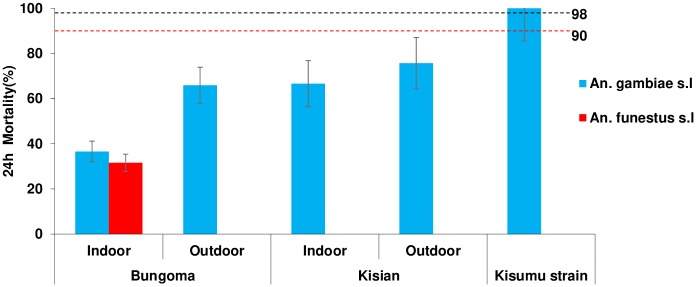
All the F1 mosquito populations tested from Bungoma and Kisian showed remarkable resistance to deltamethrin, with mortality rates ranging from 32–76% (Fig 3). Reduced mortality was observed for F1 progeny of Anopheles gambiae s.l resting indoors (37%, 95% CI: 34–39) than outdoors (67%, 95% CI: 62–69) in Bungoma. In Kisian the F1 progeny of Anopheles gambiae s.l resting indoors had lower mortality rates (67%, 95% CI: 61–73) than outdoors (76%, 95% CI: 71–80). Though the levels of deltamethrin resistance observed were higher for mosquitoes resting indoors compared to outdoors across the sites, there was no significant difference between the means (F3, 28 = 1.391, p < 0.266). The mortality rate for F1 progeny of Anopheles funestus resting indoors from Bungoma was 32% (95% CI: 28–35). Due to technical difficulties in raising An. funestus and the small numbers collected resting outdoors, susceptibility test was not done in Kisian and for the outdoor population from Bungoma. The An. gambiae s.s Kisumu susceptible laboratory strain showed 100% mortality.
Fig 3. Mortality rates of indoor and outdoor resting Anopheles gambiae s.l and An. funestus F1 progeny in standard WHO tube bioassays after exposure to 0.05% deltamethrin test papers and 24 hr recovery period.
Dotted lines represent upper (98%) and lower (90%) cut-offs for WHO classifications; values above the upper line indicate susceptibility and values below the lower red line indicate resistance (WHO, 2016). Error bars for the mean are shown.
Target site genotyping
In total 693 Anopheles, gambiae s.l samples were genotyped for the presence of Vgsc-1014S and 1014F mutations. In Bungoma, overall the frequency of Vgsc-1014S was 88% (290/328) and 6% (20/328) for Vgsc-1014F in An. gambiae, whereas only Vgsc-1014S was observed in An. arabiensis with a low frequency of 4% (2/52) (Table 1). The frequency of Vgsc-1014S and 1014F was 90 (177/195) vs 9% (20/195) respectively for indoor resting An. gambiae. Only Vgsc-1014S was observed for outdoor collections with a frequency of 85% (113/133). The Vgsc-1014S was the only kdr mutation observed in An. arabiensis resting indoors (10%, 2/20) and was not detected in the outdoor resting collections (S2 Table). In Kisian, the frequency of Vgsc-1014S in An. gambiae was 70% (89/125) and that of 1014F was 1% (2/125), whereas only Vgsc-1014S was observed in An. arabiensis with a frequency of 18% (36/188). The frequency of Vgsc-1014S mutation for An. gambiae resting indoors was 72% (78/107) and 61% (11/18) for outdoor collections. The same mutation was present in An. arabiensis collected from indoors 18% (20/110) and outdoors 18% (16/78). The Vgsc-1014F was only observed in An. gambiae caught resting indoors at a low frequency of 1%.
Table 1. Frequency of Kdr mutations in An. gambiae s.s and An. arabiensis populations of western Kenya.
| Site | Species | Location | An. gambiae s.s | An. arabiensis | ||||
|---|---|---|---|---|---|---|---|---|
| N tested | 1014S | 1014F | N tested | 1014S | 1014F | |||
| Bungoma | Indoor | 195 | 90 | 9 | 20 | 10 | 0 | |
| Outdoor | 133 | 85 | 0 | 32 | 0 | 0 | ||
| An. gambiae | 328 | 86 | 6 | |||||
| An. arabiensis | 52 | 3.8 | 0 | |||||
| Kisian | Indoor | 107 | 72 | 1 | 110 | 18 | 0 | |
| Outdoor | 18 | 61 | 0 | 78 | 18 | 0 | ||
| An. gambiae | 125 | 70 | 1 | |||||
| An. arabiensis | 188 | 18 | 0 | |||||
Blood meal sources
A total of 857 blood-fed (719 An. gambiae s.l and 138 An. funestus) mosquito specimens were analysed for blood meal origins (Table 2). An. funestus was the most anthropophagic species and An. arabiensis the least from both sites. In Bungoma, the human blood index (HBI) for An. gambiae, An. arabiensis and An. funestus caught resting indoors were 65% (273/422), 25% (6/24) and 75% (83/111) respectively. For outdoor resting An. gambiae, An. arabiensis and An. funestus the HBI was 46% (18/39), 25% (2/8) and 75% (3/4) respectively. In Kisian, the overall HBI of An. gambiae, An. arabiensis and An. funestus was 60, 7 and 83% respectively. The HBI for An. gambiae resting outdoors was 100% (3/3 and 60% (46/77) for indoor collections. The HBI for indoor and outdoor resting An. arabiensis was 7% (8/114) vs 6% (2/32). The HBI of An. funestus resting indoors was 83% (19/23).
Table 2. Blood meal origins of Anopheline mosquitoes collected from indoor and outdoor in Bungoma and Kisian, western Kenya.
| Site | Blood-meal origins | An.gambiae s.s | An. arabiensis | An. funestus | |||
|---|---|---|---|---|---|---|---|
| Indoor | Outdoor | Indoor | Outdoor | Indoor | Outdoor | ||
| Bungoma | Number tested | 422 | 39 | 24 | 8 | 111 | 4 |
| Human | 229 (54) | 13 (33) | 6 (25) | 1 (13) | 72 (65) | 3 (75) | |
| Bovine | 60 (14) | 9 (23) | 16 (67) | 3 (38) | 14 (13) | 1 (25) | |
| Human+Bovine | 31 (7) | 4 (10) | 0 | 1 (13) | 9 (8) | 0 | |
| Other | 24 (6) | 4 (1) | 2 (8) | 1 (13) | 3 (3) | 0 | |
| Un-identified | 78 (19) | 9 (23) | 0 | 2 (25) | 13 (12) | 0 | |
| HBI | 65 | 46 | 25 | 25 | 75 | 75 | |
| BBI | 22 | 36 | 67 | 63 | 21 | 25 | |
| Kisian | Number tested | 77 | 3 | 114 | 32 | 23 | 0 |
| Human | 42 (56) | 2 (67) | 6 (5) | 0 | 18 (78) | 0 | |
| Bovine | 26 (34) | 0 | 104 (91) | 29 (91) | 4 (17) | 0 | |
| Human+Bovine | 3 (4) | 1 (33) | 2 (2) | 1 (3) | 1 (4) | 0 | |
| Other | 4 (5) | 0 | 1 (1) | 2 (6) | 1 (4) | ||
| Un-identified | 2 (3) | 0 | 1 (1) | 0 | 0 | 0 | |
| HBI | 60 | 100 | 7 | 6 | 83 | 0 | |
| BBI | 40 | 33 | 93 | 97 | 22 | 0 | |
Others: dog, goat, pig, or multi-source excluding human+bovine
HBI, human blood Index; BBI, bovine blood index
Sporozoites infection rates
A total of 1,517 samples comprising of 1,156 An. gambiae s.l and 361 An. funestus specimens were tested for Plasmodium falciparum Circumsporozoite (CSP) (Table 3). Ninety-one samples (90 Bungoma and 1 Kisian) tested positive giving an overall infection rate of 8% in Bungoma and 0.3% in Kisian. Overall, the sporozoite rate was higher indoors (9%, 82/956) than outdoors (4%, 8/190) in Bungoma, whereas in Kisian the sporozoite rate was 0.3%, 1/332) indoors. None of the samples collected outdoors in Kisian tested positive (n = 73). The sporozoite for An. funestus resting indoors was 11% (34/311). None of An. funestus collected outdoors was positive. The sporozoite rate for indoor resting An. gambiae was 8% (47/618) and outdoors at 5% (7/148). For An. arabiensis caught resting indoors, the sporozoite rate was 4% (1/27) and 3% (1/35) for outdoors. In Kisian, only 1/112 (1%) An. gambiae collected from indoor was CSP positive. No CSP positives were detected in An. arabiensis and An. funestus resting indoors and outdoors in Kisian. The overall entomological inoculation rates (EIRs) of the three vector species from indoor resting collections and outdoor in Bungoma was 66 and 10 infective bites/person/year respectively. In Kisian the overall EIR from indoor collections was 1.2 infective bites/person/year.
Table 3. Sporozoite rates of Anopheles mosquitoes from indoor and outdoor collections in Bungoma and Kisian, western Kenya.
| Site | Season | Location | An. gambiae s.s | An. arabiensis | An. funestus | |||
|---|---|---|---|---|---|---|---|---|
| N tested | Pf +ve | N tested | Pf +ve | N tested | Pf +ve | |||
| Bungoma | Indoor | 618 | 47(8) | 27 | 1(4) | 311 | 34(11) | |
| Outdoor | 148 | 7(5) | 35 | 1(3) | 7 | 0 | ||
| Dry | 290 | 21(7) | 26 | 0 | 100 | 18(18) | ||
| Rainy | 476 | 33(7) | 36 | 2(6) | 218 | 17(8) | ||
| Kisian | Indoor | 112 | 1(1) | 147 | 0 | 73 | 0 | |
| Outdoor | 16 | 0 | 54 | 0 | 3 | 0 | ||
| Dry | 41 | 1(2) | 127 | 0 | 47 | 0 | ||
| Rainy | 87 | 0 | 74 | 0 | 29 | 0 | ||
Pf, Plasmodium falciparum
+ve, Positive
Discussion
Given the widespread occurrence of pyrethroid insecticide resistance in malaria vectors in western Kenya [17,19,42], little is known about the behavioural response of these mosquito populations to the wide use of LLINs. Evidence has shown that successful malaria elimination strategies require vector control interventions that target the changing vector behaviour [43]. Overall, this study investigated the behavioral heterogeneity of malaria vectors for resting behavior, feeding choices and infection rates in the context of increased use of LLINs. The study revealed high resting densities, infection rates and insecticide resistance indoors compared to outdoors.
Of the three major malaria vectors in western Kenya, An. gambiae was the major vector in Bungoma followed by An. funestus. In Kisian An. arabiensis was predominant species followed by An. gambiae. The predominance of the two malaria vectors in the two different ecological settings is consistent with previous studies [16,44,45] observing increased frequency of An. gambiae s.s at sites further away from the lake Victoria basin and increase in the frequency of An. arabiensis at sites around the lake basin. There was a rise in the abundance of An. funestus s.s in Bungoma, a phenomena that has been observed previously in some regions in western Kenya [20]. Despite the high coverage and usage of long-lasting insecticidal nets (LLIN) in the region, increased indoor resting tendency of An. gambiae and An. funestus was observed, while An. arabiensis was found mostly resting outdoors. The variation in the relative frequency and behaviour of the three vectors has been observed earlier from the region [23,46]. It is worth noting that, the proportion of An. arabiensis was higher during the dry season in Kisian (lowland). This vector has been shown to survives best in areas with lower relative humidy and high temperatures and therefore prefers the lowland areas with such climates compared to the highland areas. The dry season also has low relative humidity and high temperatures [47].
This study observed reduced susceptibility of the F1 progeny of An. funestus and An. gambiae s.l captured resting indoors compared to those resting outdoors to deltamethrin insecticide. Earlier studies from western Kenya have reported increasing phenotypic resistance of An. gambiae s.l to pyrethroids [16,45,48,49]. Few studies have reported on An. funestus insecticide resistance[50] probably because of difficulties associated with rearing the larvae. The frequency of Vgsc-1014S and Vgsc-1014F from indoors resting An. gambiae was higher than outdoors, from all sites. The Vgsc-1014F mutation was only detected in An. gambiae resting indoors, this mutation was absent in the mosquitoes collected outdoors. This might be that mosquitoes with some mutations are able to rest indoors in the presence of insecticides for vector control. Previous studies have observed the occurrence of Vgsc-1014F at low frequencies in East Africa including Kenya [42,51,52]. The presence of Vgsc-1014F in indoor resting mosquitoes may be of particular concern given that the mutation has been found to be strongly associated with pyrethroid resistance in West Africa [53]. The presence of kdr mutations at Bungoma where it was first detected in Kenya in An. gambiae [16] and now at the lowland site of Kisian indicate the widespread occurrence of the mutations among the An. gambiae population. The increased resistance level could be a result of selection pressure from insecticides used in vector control such as the widespread use of LLINs leading to the selection of resistant strains [6]. Some studies have shown a relationship between the spread of kdr alleles with the use of LLINs [54–56]. The extensive use of agriculture insecticides may also contribute to the occurrence of new mutations to existing insecticides [57–61].
Even though we observed high frequencies of Vgsc-1014S in An. gambiae, the allele was at low frequency in An. arabiensis, with a higher frequency of Vgsc-1014S, detected for indoor resting individuals than outdoors. Most recently, the presence of kdr mutations in An. arabiensis from the lowlands of western Kenya has been reported [17,49]. The low kdr frequency observed in An. arabiensis could be due to the reduced insecticide selection pressure as they resort to feed and rest outdoors in the absence of insecticides, unlike An. gambiae that feeds and rests indoors. The high frequency of kdr mutations, behavioural resilience and an increased proportion of An. arabiensis resting outdoors could all raise further concerns on the future utility of the current indoor interventions.
Sporozoite infection rates were high in An. funestus and An. gambiae collected from Bungoma, with An. funestus showing considerably higher sporozoite rates than the other species. These findings are in agreement with previous studies that observed high sporozoite rates in An. funestus and An. gambaie, implying the importance of the two vectors in malaria transmission in the region [20,46]. The higher sporozoite rates in An. funestus in this study further indicates its reemergence and as one of the primary important vector in malaria transmission in the region. The blood meal analysis showed a large proportion of the two species preferred feeding on humans than animals, with An. funestus observed to be highly anthropophagic in the region. This consistency in host choice has been frequently observed in An. funestus in Kenya and other parts of Africa [46,62,63]. This human-host choice and higher indoor resting proportions of An. funestus together with increased resistance poses a great concern in malaria elimination efforts due to its efficiency in transmitting malaria. These findings confirm earlier reports from other regions in western Kenya that have documented the re-emergence of An. funestus and its role in malaria transmission [20,64]. The infection rates were higher for vectors collected indoors than outdoors, suggesting ongoing malaria transmission regardless of the use of LLINs. Earlier reports from western Kenya have shown increased EIR for indoor collected mosquitoes [46]. Some studies have linked the rebound of malaria in western Kenya with increasing insecticide resistance after high coverage of LLINs [54,56,65]. The observed sporozoite infection rates outdoors might be attributed to changing in the biting behaviour of malaria vectors as some vectors could be feeding on humans when they are active and unprotected outdoors [66]. Complementary malaria control intervenions are thus, needed to control outdoor resting and biting mosquitoes, as the current tools only target indoor resting and biting mosquitoes.
Despite the low sporozoite rates of An. arabiensis reported in this study, its importance in outdoor malaria transmission should not be taken for granted due to its opportunistic behavior, as the vector could continue with transmission outdoors in the region, which is a major threat to effective malaria vector control.
Conclusion
The study shows high densities of An. gambiae and An. funestus resting indoors despite the use of indoor interventions with the increasing importance of An. funestus in sustaining malaria transmission in western Kenya highlands. An. arabiensis were more outdoors than indoors. This behavioural plasticity increases its survival and potential in continuing residual transmission after the main endophilic and endophagic vectors have been reduced by the interventions. The Vgsc-1014S and Vgsc-1014F mutations were observed at high frequencies in An. gambiae resting indoors. This calls for further screening of other resistance mutations in this population for better resistance management. Sporozoite rates were higher indoors than outdoors, showing that transmission occurs more indoors than outdoors in these sites. Insecticide resistance management strategies and/or new vector control interventions that may not be insecticide based are needed in western Kenya to reduce malaria transmission.
Supporting information
(DOCX)
(DOCX)
Acknowledgments
The authors wish to thank the villagers and community leaders in Bungoma and Kisian for their permission to collect mosquitoes in their houses. We acknowledge the Entomology Laboratory at Kenya Medical Research Institute, Kisumu for providing technical and laboratory space for the study. The permission to publish this study was granted by the director of Kenya Medical Research Institute.
Abbreviations
- BBI
bovine blood index
- CSP
circumsporozoite protein
- EIR
entomological inoculation rate
- ELISA
enzyme-linked immunosorbent assay
- F1
first generation offspring
- HBI
human blood index
- IRS
indoor residual spray
- KDR
Knockdown resistant gene
- LLINs
long-lasting insecticidal nets
- PCR
polymerase chain reaction
- PSC
pyrethrum spray catch
- Vgsc
voltage-gated sodium channel
Data Availability
All relevant data are within the paper and its two Supporting Information files.
Funding Statement
This study was supported by grants from the National Institute of Health (R01 A1123074, U19 AI129326, R01 AI050243, D43 TW001505). There was no additional external funding received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO (2019) World Malaria Report 2018. Geneva: World Health Organisation.
- 2.Hamel MJ, Adazu K, Obor D, Sewe M, Vulule J, et al. (2011) A reversal in reductions of child mortality in western Kenya, 2003–2009. Am J Trop Med Hyg 85: 597–605. 10.4269/ajtmh.2011.10-0678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatt S, Weiss D, Cameron E, Bisanzio D, Mappin B, et al. (2015) The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526: 207 10.1038/nature15535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO (2018) World Malaria Report 2018. Geneva: World Health Organization.
- 5.Zhou G, Afrane YA, Vardo-Zalik AM, Atieli H, Zhong D, et al. (2011) Changing patterns of malaria epidemiology between 2002 and 2010 in Western Kenya: the fall and rise of malaria. PloS one 6: e20318 10.1371/journal.pone.0020318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ranson H, Lissenden N (2016) Insecticide resistance in African Anopheles mosquitoes: A worsening situation that needs urgent action to mantain malaria control. Parasites Vectors 32: 187–196. [DOI] [PubMed] [Google Scholar]
- 7.Russell TL, Beebe NW, Cooper R, Lobo NF, Burkot T (2013) Successful malaria elimination strategies require interventions that target changing vector behaviours. Malaria journal 12: 56 10.1186/1475-2875-12-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen JM, Smith DL, Cotter C, Ward A, Yamey G, et al. (2012) Malaria resurgence: a systematic review and assessment of its causes. Malaria journal 11: 122 10.1186/1475-2875-11-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.PMI (2019) Kenya Malaria Operational Plan FY 2019.
- 10.Ototo EN, Mbugi JP, Wanjala CL, Zhou G, Githeko AK, et al. (2015) Surveillance of malaria vector population density and biting behaviour in western Kenya. Malaria journal 14: 244 10.1186/s12936-015-0763-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siegert PY, Walker E, Miller JR (2009) Differential behavioral responses of Anopheles gambiae (Diptera: Culicidae) modulate mortality caused by pyrethroid-treated bednets. Journal of economic entomology 102: 2061–2071. 10.1603/029.102.0607 [DOI] [PubMed] [Google Scholar]
- 12.Killeen GF, Govella NJ, Lwetoijera DW, Okumu FO (2016) Most outdoor malaria transmission by behaviourally-resistant Anopheles arabiensis is mediated by mosquitoes that have previously been inside houses. Malaria journal 15: 225 10.1186/s12936-016-1280-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durnez L, Coosemans M (2013) Residual transmission of malaria: an old issue for new approaches Anopheles mosquitoes: new insights into malaria vectors/Manguin Sylvie. pp. 671–704. [Google Scholar]
- 14.Ranson H, Abdallah H, Badolo A, Guelbeogo WM, Kerah-Hinzoumbé C, et al. (2009) Insecticide resistance in Anopheles gambiae: data from the first year of a multi-country study highlight the extent of the problem. Malaria Journal 8: 299 10.1186/1475-2875-8-299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO (2017) World Malaria Report 2017 Geneva: World Health Organization.
- 16.Ochomo E, Bayoh MN, Brogdon WG, Gimnig JE, Ouma C, et al. (2012) Pyrethroid resistance in Anopheles gambiae s.s. and Anopheles arabiensis in western Kenya: phenotypic, metabolic and target site characterizations of three populations. Medical and Veterinary Entomology 27: 156–164. 10.1111/j.1365-2915.2012.01039.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wanjala CL, Mbugi JP, Ototo E, Gesuge M, Afrane YA, et al. (2015) Pyrethroid and DDT resistance and organophosphate susceptibility among Anopheles spp. mosquitoes, western Kenya. Emerging infectious diseases 21: 2178 10.3201/eid2112.150814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ndenga BA, Mulaya NL, Musaki SK, Shiroko JN, Dongus S, et al. (2016) Malaria vectors and their blood-meal sources in an area of high bed net ownership in the western Kenya highlands. Malaria journal 15: 76 10.1186/s12936-016-1115-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ochomo E, Bayoh NM, Kamau L, Atieli F, Vulule J, et al. (2014) Pyrethroid susceptibility of malaria vectors in four Districts of western Kenya Parasites Vectors 7: 310 10.1186/1756-3305-7-310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCann RS, Ochomo E, Bayoh MN, Vulule JM, Hamel MJ, et al. (2014) Reemergence of Anopheles funestus as a vector of Plasmodium falciparum in western Kenya after long-term implementation of insecticide-treated bed nets. Am J Trop Med Hyg 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindblade KA, Gimnig JE, Kamau L, Hawley WA, Odhiambo F, et al. (2006) Impact of sustained use of insecticide-treated bednets on malaria vector species distribution and culicine mosquitoes. Journal of Medical Entomology 43: 428–432. 10.1603/0022-2585(2006)043[0428:iosuoi]2.0.co;2 [DOI] [PubMed] [Google Scholar]
- 22.Bayoh MN, Mathias DK, Odiere MR, Mutuku FM, Kamau L, et al. (2010) Anopheles gambiae: historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province, Kenya. Malaria J 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bayoh MN, Walker ED, Kosgei J, Ombok M, Olang GB, et al. (2014) Persistently high estimates of late night, indoor exposure to malaria vectors despite high coverage of insecticide treated nets. Parasites & Vectors 7: 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takken W (2002) Do insecticide-treated bednets have an effect on malaria vectors? Tropical Medicine & International Health 7: 1022–1030. [DOI] [PubMed] [Google Scholar]
- 25.Mbogo CNM, Baya NM, Ofulla AVO, Githure JI, Snow RW (1996) The impact of permethrin-impregnated bednets on malaria vectors of the Kenyan coast. Medical and veterinary entomology 10: 251–259. 10.1111/j.1365-2915.1996.tb00739.x [DOI] [PubMed] [Google Scholar]
- 26.Davidson JR, Sukowati S, Asih PBS, Syafruddin D, Baskin RN, et al. (2018) Using barrier screens to characterize mosquito composition, flight activity, and abdominal status in South Lampung, Indonesia. Parasites & vectors 11: 440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russell TL, Govella NJ, Azizi S, Drakeley CJ, Kachur SP, et al. (2011) Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malaria J 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonizzoni M, Afrane Y, Dunn WA, Atieli FK, Zhou G (2012) Comparative Transcriptome Analyses of Deltamethrin-Resistant and -Susceptible Anopheles gambiae Mosquitoes from Kenya by RNA-Seq. PLoS ONE 7(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muirhead-Thomson R (1958) A pit shelter for sampling outdoor mosquito populations. Bulletin of the World Health Organization 19: 1116 [PMC free article] [PubMed] [Google Scholar]
- 30.Odiere M, Bayoh M, Gimnig J, Vulule J, Irungu L, et al. (2007) Sampling outdoor, resting Anopheles gambiae and other mosquitoes (Diptera: Culicidae) in western Kenya with clay pots. Journal of medical entomology 44: 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van den Bijllaardt W, ter Braak R, Shekalaghe S, Otieno S, Mahande A, et al. (2009) The suitability of clay pots for indoor sampling of mosquitoes in an arid area in northern Tanzania. Acta tropica 111: 197–199. 10.1016/j.actatropica.2009.04.003 [DOI] [PubMed] [Google Scholar]
- 32.Gillies MT, Coetzee M (1987) Supplement to the Anophelinae of Africa South of the Sahara (Afrotropical region). Publication of South African Institute of Medical Research 55: 143. [Google Scholar]
- 33.WHO (2013) Test procedures for insecticide resistance monitoring in malaria vector mosquitoes Geneva: World Health Organization. [Google Scholar]
- 34.Scott JA, Brogdon WG, Collins FH (1993) Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. The American journal of tropical medicine and hygiene 49: 520–529. 10.4269/ajtmh.1993.49.520 [DOI] [PubMed] [Google Scholar]
- 35.Cohuet A, Simard F, TOTO J-C, Kengne P, COETZEE M, et al. (2003) Species identification within the Anopheles funestus group of malaria vectors in Cameroon and evidence for a new species. The American journal of tropical medicine and hygiene 69: 200–205. [PubMed] [Google Scholar]
- 36.Collins FH, Mendez MA, Rasmussen MO, Mehaffey PC, Besansky NJ, et al. (1987) A ribosomal RNA gene probe differentiates member species of the Anopheles gambiae complex. The American journal of tropical medicine and hygiene 37: 37–41. 10.4269/ajtmh.1987.37.37 [DOI] [PubMed] [Google Scholar]
- 37.Wirtz RA, Ballou WR, Schneider I, Chedid L, Gross MJ, et al. (1987) Plasmodium falciparum:Immunogenicity of circumsporozoite protein constructs produced inEscherichia coli. Experimental Parasitology 63: 166–172. 10.1016/0014-4894(87)90158-5 [DOI] [PubMed] [Google Scholar]
- 38.Kent RJ, Thuma PE, Mharakurwa S, Norris DE (2007) Seasonality, blood feeding behavior, and transmission of Plasmodium falciparum by Anopheles arabiensis after an extended drought in southern Zambia. The American journal of tropical medicine and hygiene 76: 267–274. [PMC free article] [PubMed] [Google Scholar]
- 39.Bass C, Nikou D, Donnelly MJ, Williamson MS, Ranson H, et al. (2007) Detection of knockdown resistance (kdr) mutations in Anopheles gambiae: a comparison of two new high-throughput assays with existing methods Malaria Journal 6 111 10.1186/1475-2875-6-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garrett-Jones C (1964) The human blood index of malaria vectors in relation to epidemiological assessment. Bulletin of the World Health Organization 30: 241 [PMC free article] [PubMed] [Google Scholar]
- 41.Pappa V, Reddy M, Overgaard HJ, Abaga S, Caccone A (2011) Estimation of the human blood index in malaria mosquito vectors in Equatorial Guinea after indoor antivector interventions. The American journal of tropical medicine and hygiene 84: 298–301. 10.4269/ajtmh.2011.10-0463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ochomo E, Subramaniam K, Kemei B, Rippon E, Bayoh NM, et al. (2015) Presence of the knockdown resistance mutation, Vgsc-1014F in Anopheles gambiae and An. arabiensis in western Kenya. Parasites & Vectors 8: 616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Russell TL, Beebe NW, Cooper RD, Lobo NF, Burkot TR (2013) Successful malaria elimination strategies require interventions that target changing vector behaviours. Malar J 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ndenga B, Githeko A, Omukunda E, Munyekenye G, Atieli H, et al. (2006) Population Dynamics of Malaria Vectors in Western Kenya Highlands. Journal of Medical Entomology 43: 200–206. 10.1603/0022-2585(2006)043[0200:pdomvi]2.0.co;2 [DOI] [PubMed] [Google Scholar]
- 45.Wanjala CL, Mbugi JP, Ototo E, Gesuge M, Afrane Y, et al. (2015) Pyrethroid and DDT resistance and organophosphate susceptibility among Anopheles spp. mosquitoes from Western Kenya. Emerging infectious Diseases 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Degefa T, Yewhalaw D, Zhou G, Lee M-c, Atieli H, et al. (2017) Indoor and outdoor malaria vector surveillance in western Kenya: implications for better understanding of residual transmission. Malaria journal 16: 443 10.1186/s12936-017-2098-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Afrane YA, Zhou G, Lawson BW, Githeko AK, Yan G (2007) Life-table analysis of Anopheles arabiensis in western Kenya highlands: effects of land covers on larval and adult survivorship. The American journal of tropical medicine and hygiene 77: 660–666. [PubMed] [Google Scholar]
- 48.Mathias D, Ochomo E, Atieli F, Ombok M, Bayoh N, et al. (2011) Spatial and temporal variation in the kdr allele L1014S in Anopheles gambiae s.s. and phenotypic variability in susceptibility to insecticides in Western Kenya. Malaria Journal 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hemming-Schroeder E, Strahl S, Yang E, Nguyen A, Lo E, et al. (2018) Emerging pyrethroid resistance among Anopheles arabiensis in Kenya. The American journal of tropical medicine and hygiene 98: 704–709. 10.4269/ajtmh.17-0445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kawada H, Dida GO, Ohashi K, Komagata O, Kasai S, et al. (2011) Multimodal Pyrethroid Resistance in Malaria Vectors, Anopheles gambiae s.s., Anopheles arabiensis, and Anopheles funestus s.s. in Western Kenya. PLOS ONE 6: e22574 10.1371/journal.pone.0022574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kabula B, Kisinza W, Tungu P, Ndege C, Batengana B, et al. (2014) Co-occurrence and distribution of East (L1014S) and West (L1014F) African knock- down Magesa resistance in Anopheles gambiae sensu lato population of Tanzania. Trop Med Int Health 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verhaeghen K, Bortel VW, Roelants P, Backeljau T, Coosemans M (2006) Detection of the East and West African kdr mutation in Anopheles gambiae and Anopheles arabiensis from Uganda using a new assay based on FRET/Melt Curve analysis. Malaria Journal 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lynd A, Weetman D, Barbosa S, Egyir Yawson A, Mitchell S, et al. (2010) Field, genetic, and modeling approaches show strong positive selection acting upon an insecticide resistance mutation in Anopheles gambiae s.s. Mol Biol Evol 27: 1117–1125. 10.1093/molbev/msq002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ndiath MO, Mazenot C, Sokhna C, Trape J-F (2014) How the malaria vector Anopheles gambiae adapts to the use of insecticide-treated nets by African populations. PloS one 9: e97700 10.1371/journal.pone.0097700 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55.Ochomo EO, Bayoh NM, Walker ED, Abongo BO, Ombok MO, et al. (2013) The efficacy of long-lasting nets with declining physical integrity may be compromised in areas with high levels of pyrethroid resistance. Malaria Journal 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trape JF, Tall A, Diagne N, Ndiath O, Ly AB, et al. (2011) Malaria morbidity and pyrethroid resistance after the introduction of insecticide-treated bednets and artemisinin-based combination therapies: a longitudinal study. The Lancet infectious diseases 11: 925–932. 10.1016/S1473-3099(11)70194-3 [DOI] [PubMed] [Google Scholar]
- 57.Diabate A, Baldet T, Chandre F, Akogbeto M, Guiguemde TR, et al. (2002) The role of agricultural use of insecticides in resistance to pyrethroids in Anopheles gambiae s.l. in Burkina Faso. American Journal of Tropical Medicine and Hygiene 67 617–622. 10.4269/ajtmh.2002.67.617 [DOI] [PubMed] [Google Scholar]
- 58.Luc DS, Benoit A, Laurette D, Michel M (2016) Indirect evidence that agricultural pesticides select for insecticide resistance in the malaria vector Anopheles gambiae. Journal of Vector Ecology 41: 34–40. 10.1111/jvec.12191 [DOI] [PubMed] [Google Scholar]
- 59.Lines JD (1988) Do agricultural insecticides select for insecticide resistance in mosquitoes? A look at the evidence. Parasitology today 4: 17–20. [DOI] [PubMed] [Google Scholar]
- 60.Reid MC, McKenzie FE (2016) The contribution of agricultural insecticide use to increasing insecticide resistance in African malaria vectors. Malaria journal 15: 107 10.1186/s12936-016-1162-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wanjala CL, Zhou G, Mbugi J, Simbauni J, Afrane YA, et al. (2015) Insecticidal decay effects of long-lasting insecticide nets and indoor residual spraying on Anopheles gambiae and Anopheles arabiensis in Western Kenya. Parasites & vectors 8: 588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mwangangi JM, Mbogo CM, Orindi BO, Muturi EJ, Midega JT, et al. (2013) Shifts in malaria vector species composition and transmission dynamics along the Kenyan coast over the past 20 years. Malaria Journal 12: 13 10.1186/1475-2875-12-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tanga M, Ngundu W, Tchouassi P (2011) Daily survival and human blood index of major malaria vectors associated with oil palm cultivation in Cameroon and their role in malaria transmission. Tropical Medicine & International Health 16: 447–457. [DOI] [PubMed] [Google Scholar]
- 64.Ogola EO, Fillinger U, Ondiba IM, Villinger J, Masiga DK, et al. (2018) Insights into malaria transmission among Anopheles funestus mosquitoes, Kenya. Parasites & vectors 11: 577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gimnig JE, Vulule JM, Lo TQ, Kamau L, Kolczak MS, et al. (2003) Impact of permethrin-treated bed nets on entomologic indices in an area of intense year-round malaria transmission. The American journal of tropical medicine and hygiene 68: 16–22. [PubMed] [Google Scholar]
- 66.Killeen GF (2014) Characterizing, controlling and eliminating residual malaria transmission. Malaria Journal 13: 330 10.1186/1475-2875-13-330 [DOI] [PMC free article] [PubMed] [Google Scholar]