Abstract
Amblyomma americanum ticks transmit more than a third of human tick-borne disease (TBD) agents in the United States. Tick saliva proteins are critical to success of ticks as vectors of TBD agents, and thus might serve as targets in tick antigen-based vaccines to prevent TBD infections. We describe a systems biology approach to identify, by LC-MS/MS, saliva proteins (tick = 1182, rabbit = 335) that A. americanum ticks likely inject into the host every 24 h during the first 8 days of feeding, and towards the end of feeding. Searching against entries in GenBank grouped tick and rabbit proteins into 27 and 25 functional categories. Aside from housekeeping-like proteins, majority of tick saliva proteins belong to the tick-specific (no homology to non-tick organisms: 32%), protease inhibitors (13%), proteases (8%), glycine-rich proteins (6%) and lipocalins (4%) categories. Global secretion dynamics analysis suggests that majority (74%) of proteins in this study are associated with regulating initial tick feeding functions and transmission of pathogens as they are secreted within 24–48 h of tick attachment. Comparative analysis of the A. americanum tick saliva proteome to five other tick saliva proteomes identified 284 conserved tick saliva proteins: we speculate that these regulate critical tick feeding functions and might serve as tick vaccine antigens. We discuss our findings in the context of understanding A. americanum tick feeding physiology as a means through which we can find effective targets for a vaccine against tick feeding.
Author summary
The lone star tick, Amblyomma americanum, is a medically important species in US that transmits 5 of the 16 reported tick-borne disease agents. Most recently, bites of this tick were associated with red meat allergies in humans. Vaccination of animals against tick feeding has been shown to be a sustainable and an effective alternative to current acaricide based tick control method which has several limitations. The pre-requisite to tick vaccine development is to understand the molecular basis of tick feeding physiology. Toward this goal, this study has identified proteins that A. americanum ticks inject into the host at different phases of its feeding cycle. This data set has identified proteins that A. americanum inject into the host within 24–48 h of feeding before it starts to transmit pathogens. Of high importance, we identified 284 proteins that are present in saliva of other tick species, which we suspect regulate important role(s) in tick feeding success and might represent rich source target antigens for a tick vaccine. Overall, this study provides a foundation to understand the molecular mechanisms regulating tick feeding physiology.
Introduction
Ticks and tick-borne diseases (TBDs) have been on the rise and have greatly impacted human and veterinary medicine. Ticks have gained the attention in public health policy with a recent publication that advocated for One Health solutions listing 17 human TBDs among sources of human health concerns [1]. Moreover, the dramatic rise related to ticks and TBDs have caught the attention of United States (US) lawmakers, as shown in the 21st Century Cures Act of 2016, which created the TBD Working Group. Under the Cures Act, the TBD Working Group was tasked with evaluating the impact of TBDs and required research to find solutions (https://www.hhs.gov/ash/advisory-committees/tickbornedisease/index.html). Likewise, six of the 23 human vector-borne diseases that are listed by the World Health Organization are tick-borne that include Crimean-Congo haemorrhagic fever, Lyme disease, relapsing fever, rickettsial diseases (spotted fever and Q fever), tick-borne encephalitis, and tularemia (http://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases). In the US, Amblyomma americanum, the lone star tick is among one of the tick species of medical and veterinary health significance.
A. americanum is a geographically expanding tick species [2] that is involved in transmission of multiple human and animal disease agents. In public health, A. americanum is the principal vector for Ehrlichia chaffensis, the causative agent of human monocytic ehrlichiosis [3], and E. ewingii, which also causes ehrlichiosis, referred to as human granulocytic ehrlichiosis [4–6]. This tick also transmits Francisella tularensis, the causative agent for tularemia [7, 8], a yet to be described disease agent, suspected as Borrelia lonestari, which causes Lyme disease-like symptoms referred to as southern tick-associated rash illness (STARI) [9, 10] and also an E. ruminantium-like organism referred to as the Panola Mountain Ehrlichia (PME) [11]. There is also evidence that A. americanum may transmit Rickettsia amblyommii, R. rickettsia, and R. parkeri, the causative agents to rickettsiosis to humans [12, 13]. This tick has also been reported to transmit the Heartland and Bourbon viruses to humans [14, 15]. Most recently, this tick has been shown to be responsible for causing an α-gal allergy or mammalian meat allergy (MMA) in humans upon tick bite [16]. In veterinary health, A. americanum transmits Theileria cervi to deer [17], and E. ewingii to dogs [18]. There are reports of mortality in deer fawns that were attributed to a combination of heavy A. americanum infestation and T. cervi infections [19]. In livestock production, heavy infestations were thought to cause low productivity in cattle [20, 21]. In the Southern US, A. americanum appears to be the most dominant tick species that bite humans, which has been reported to be responsible for 83% of human tick infestations [22].
The success of ticks as pests and vectors of TBD agents is facilitated by secreted tick salivary proteins that are injected into the host to regulate the tick’s evasion of host defense [23]. There is evidence that repeatedly infested animals develop immunity against tick saliva proteins and are protected against TBD transmissions such as Francisella tularensis [24], B. burgdorferi [25–27], Babesia spp. [28], Thogoto virus [29], tick-borne encephalitis virus [30] and T. parva bovis [31]. Therefore, identification of tick saliva proteins that ticks inject into the host during feeding might lead to development of tick saliva protein-based vaccines to prevent TBD infections.
The goal of this study was to utilize systems biology approach to identify proteins that A. americanum ticks injects during feeding. This study builds upon our recent findings that identified Ixodes scapularis tick saliva proteins that are secreted every 24 h during first five days of feeding [32], partial and replete fed Rhipicephalus microplus [33], and replete fed adult and nymph Haemaphysalis longicornis [34]. Others have reported proteins in saliva of replete fed R. sanguineus [35] and three and five day fed Dermacentor andersoni [36]. A related study reported proteins in Ornithodoros moubata (soft tick) identified from saliva collected after four months from feeding [37]. Most recently, the saliva proteomes of unfed I. scapularis and A. americanum exposed to different hosts have been identified [38]. In this study, we report proteins that A. americanum ticks sequentially inject into the host every 24 h during feeding. Comparison of the A. americanum tick saliva proteome in this study with other saliva proteomes of other tick species allowed us to identify tick saliva proteins that are likely utilized by multiple tick species to regulate feeding, and these might represent potential antigens for anti-tick vaccine development.
Materials and methods
Ethics statement
All experiments were done according to the animal use protocol approved by Texas A&M University Institutional Animal Care and Use Committee (IACUC) (AUP 2011–207 and 2011–189) that meets all federal requirements, as defined in the Animal Welfare Act (AWA), the Public Health Service Policy (PHS), and the Humane Care and Use of Laboratory Animals.
A. americanum tick saliva collection
A. americanum ticks were purchased from the tick rearing facility at Oklahoma State University (Stillwater, OK, USA). Routinely, ticks were fed on rabbits as previously described [39, 40]. Ticks were restricted to feed on the outer part of the ear of New Zealand rabbits with orthopedic stockinet’s glued with Kamar adhesive (Kamar Products Inc., Zionsville, IN, USA). To stimulate female A. americanum ticks to attach onto the host to start feeding and to be inseminated to complete the feeding process, male ticks (15 per ear) were pre-fed for three days prior to placing female ticks onto rabbit ears to feed. A total of 50 female A. americanum ticks (25 per ear) were placed into tick containment apparatus on each of the three rabbits and allowed to attach.
Saliva of female A. americanum tick was collected as previously described [32, 33]. Saliva was collected and pooled from 30 ticks fed for 24, 48, 72, 96, 120, 144, 168, and 192 h, respectively, ten ticks for apparently engorged but not detached from the host (BD), and six ticks for replete fed and spontaneously detached from the host (SD). Tick saliva was collected every 15–30 min intervals for a period of approximately 4 h at room temperature from ticks that were previously injected with 1–3 μL of 2% pilocarpine hydrochloride in phosphate buffered saline (PBS, pH 7.4) as published by our group [32, 38]. Protein quantification was routinely done using the Pierce BCA (bicinchoninic acid assay) Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA).
Identification of A. americanum tick saliva proteins by LC-MS/MS
A. americanum tick saliva proteins were identified from an in-solution preparation method using LC-MS/MS as previously described [32–34]. Approximately 2 μg of total tick saliva proteins (in triplicate runs) per feeding time point (24, 36, 48, 72, 96, 120, 144, 168, 192 h fed, BD, and SD) were diluted in 8 M urea/0.1 M Tris (pH 8.5), reduced with 5 mM Tris (2-carboxyethyl) phosphine hydrochloride (TCEP, Sigma-Aldrich, St Louis, MO, USA), and alkylated with 25 mM iodoaceamide (Sigma-Aldrich). Proteins were digested overnight at 37°C in 2 M urea/0.1M Tris pH 8.5, 1 mM CaCl2 with trypsin (Promega, Madison, WI, USA) with a final ratio of 1:20 (enzyme:substrate). Digestion reactions, in a final concentration of 0.15 μg/mL (per technical replicate), were quenched with formic acid (5% final concentration) and centrifuged for debris removal before peptide mixtures were analyzed by nanoflow liquid chromatography mass spectrometry using an Easy NanoLC II and a Q Exactive mass spectrometer (Thermo Fisher Scientific). The mass spectrometry proteomics data have been deposited into ProteomeXchange Consortium via the PRIDE [41] partner repository with the dataset identifier PXD014844.
Database searching of tandem mass spectra
Proteins in A. americanum tick saliva were identified according to the previously described pipeline [32–34]. To prepare the protein database used for protein identification, we extracted the coding sequences (CDS) from A. americanum transcriptomes that were assembled from Illumina sequence reads (BioProject accession # PRJNA226980) [42] using an automated pipeline in Visual Basic (Microsoft, Redmond, Washington, USA) provided Dr. Jose M. Ribeiro (NIH), based on similarities to known proteins [43]. Contigs from the assembled A. americanum transcriptome were used to identify open reading frames (ORFs) that were larger than 50 amino acids in all six frames. For functional annotation, the identified ORFs were subjected to blastp using several amino acid sequence databases downloaded from NCBI (non-redundant [nr] Acari and refseq-invertebrate), Uniprot (nr-Acari), MEROPS database [44], the GeneOntology (GO) FASTA subset [45] and the conserved domains database (CDD) of NCBI [46] containing the COG [47], PFAM [48], and SMART motifs [49]. Outputs from the blast searches were used in the classifier program in Dr. Ribeiro's visual basic program [43] to functionally categorize the identified proteins based on the best match from among all the blast screens. The functionally annotated proteins were manually validated. As a false-discovery approach to identify transcripts related to hosts, we searched the ORFs against the nr-databases from NCBI for rabbit, mouse, rat, goat, sheep, cow, monkey, and humans. CDS were extracted from blastp searches that matched with 70% identity and e-value of 1e-40. To remove redundancies, CD-HIT [50] was used to remove sequences at 98% identity. The extracted CDS (n = 110,587) were concatenated with Oryctolagus cuniculus from Uniprot (www.uniprot.org) (n = 21,148) and reverse sequences of all entries were used to identify peptides from tandem mass spectra.
Proteins were identified by first extracting the tandem mass spectra from Thermo RAW files using RawExtract 1.9.9.2 [51] and then searching against the protein database (described above) using ProLuCID in the Integrated Proteomics Pipeline Ver.5.0.1 [52]. At least two peptide matches were required to be considered a protein hit. A cutoff score was established to accept a protein false discovery rate (FDR) of 1% based on the number of decoys. Additionally, a minimum sequence length of six residues per peptide was required. Results were post processed to only accept PSMs with <10ppm precursor mass error. Finally, the protein matches from each sampled time points were concatenated into one file using the Identification Compare (IDcompare) program on IP2- Integrated Proteomics Pipeline Ver.5.0.1 [52].
Relative abundance and graphical visualization of secretion dynamics of A. americanum tick saliva proteins
Relative abundance and secretion dynamics were determined as described [32] using normalized spectral abundance factors (NSAF) that were validated as reliable in a label-free relative quantification approach [53–55]. For each functional category or individual protein, NSAF was expressed as a percent (%) of total NSAF for that time point. Percent NSAF values were normalized using Z-score statistics using the formula , where Z is the Z-score, X is the NSAF for each protein per time point, μ is the mean throughout time points, σ is the standard deviation throughout time points. Normalized percent NSAF values were used to generate heat maps using the heatmap2 function from the gplots library in R [56]. Secretion dynamics (low to high abundance) were used to assemble clusters on heat maps.
Identification of A. americanum saliva proteins found in saliva of other tick species
A. americanum tick saliva proteins in this study were searched against published tick saliva proteomes of R. microplus [32], I. scapularis [33], H. longicornis [34], R. sanguineus [35], D. andersoni [36], and O. moubata [37] using local BLASTp analysis. Databases of protein sequences reported for each tick saliva proteome were extracted from NCBI or Uniprot and screened by BLASTp using the A. americanum saliva proteome (from this study) as the query. Protein matches ≥70% identity was reported.
Results and discussion
Protein profile and abundance changes every 24 h during A. americanum tick feeding Previous studies have demonstrated that the protein profile and abundance in salivary glands of female A. americanum is dynamic and changes during the course of tick feeding [57]. However, a limitation to the previous study was that it did not inform which salivary gland proteins were secreted during feeding. To attempt at capturing changes in tick saliva protein profiles, we successfully used pilocarpine to induce and collect saliva from A. americanum ticks every 24 h during the first eight days of tick feeding as we all as from ticks that had engorged but had not detached, and replete fed ticks [32, 58]. In early feeding stages (24–72 h), A. americanum tick saliva was observed as a white flake that accumulated on the mouthparts over time and was collected every 15–30 min for 4 h by washing the mouthparts with sterile phosphate buffered saline. Tick saliva was more evident after 72 h of feeding, observed as droplets of liquid forming at the mouthparts.
Saliva collected from ticks that had fed for 24, 48, 72, 96, 120, 144, 168, and 192 h as well as ticks that were apparently engorged but were not detached from the host (BD) and replete fed (SD) was subjected to LC-MS/MS analysis for protein identification. Peptide mass spectra were searched against the combined database (described above) using the ProLuCID search engine [52]. We identified 450, 540, 419, 441, 332, 529, 478, 536, 312, and 325 tick proteins in the 10 different saliva samples (S1 Table). Similarly, we respectively identified 127, 130, 115, 147, 112, 140, 199, 198, 78, and 282 as rabbit proteins (S1 Table). The identification of 1182 tick and 335 rabbit unique proteins in tick saliva demonstrates the complexity of tick and host interactions.
Tick and rabbit proteins in A. americanum tick saliva are annotated in multiple functional categories
With redundancy removed at 98% amino acid identity levels, we have identified 1182 tick and 335 rabbit unique proteins, which we have categorized into a respective 27 (Tables 1 and 2) and 25 (Tables 3 and 4) functional categories. Data in Tables 1–4 summarizes total number of proteins (protein count) and cumulative relative abundance based on normalized spectral abundance factor (NSAF, the index for relative protein abundance [53–55]) for each functional category. Please note that Tables 1 and 3 summarizes respective tick and rabbit proteins that were identified in 24 to 120 h saliva, while Tables 2 and 4 has proteins that were identified in 144–192 h, BD and SD saliva.
Table 1. Numbers and cumulative relative abundance of tick protein classes in Amblyomma americanum saliva during 24–120 h of feeding (NSAF = normalized spectral abundance factor).
| Feeding Time Point | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 96 h | 120 h | ||||||
| Classification | Protein Count | NSAF (%) | Protein Count | NSAF (%) | Protein Count | NSAF (%) | Protein Count | NSAF (%) | Protein Count | NSAF (%) |
| antimicrobial | 6 | 4.20 | 6 | 3.30 | 5 | 6.01 | 5 | 6.15 | 5 | 6.73 |
| cytoskeletal | 42 | 4.77 | 16 | 1.32 | 25 | 2.13 | 14 | 1.86 | 6 | 0.71 |
| detoxification | 19 | 2.16 | 15 | 0.71 | 12 | 0.72 | 15 | 0.89 | 3 | 0.18 |
| evasin | 0 | 0.00 | 6 | 0.74 | 2 | 0.24 | 6 | 0.68 | 7 | 1.67 |
| extracellular matrix | 20 | 1.81 | 11 | 1.20 | 17 | 1.48 | 11 | 0.64 | 6 | 0.37 |
| glycine rich | 23 | 2.41 | 43 | 5.81 | 44 | 9.82 | 41 | 4.86 | 27 | 6.83 |
| heme/iron binding | 16 | 20.20 | 16 | 7.48 | 15 | 15.27 | 16 | 12.35 | 9 | 5.81 |
| immune-related | 11 | 2.96 | 9 | 2.28 | 8 | 4.12 | 9 | 4.47 | 7 | 3.54 |
| ixodegrin | 1 | 0.46 | 5 | 0.82 | 1 | 0.84 | 2 | 0.47 | 2 | 0.62 |
| lipocalin | 8 | 0.74 | 17 | 2.90 | 2 | 0.55 | 6 | 1.01 | 3 | 4.39 |
| metabolism, amino acid | 3 | 0.08 | 0 | 0.00 | 1 | 0.02 | 0 | 0.00 | 0 | 0.00 |
| metabolism, carbohydrate | 11 | 0.57 | 7 | 0.22 | 8 | 0.36 | 9 | 0.77 | 3 | 0.06 |
| metabolism, energy | 18 | 1.22 | 17 | 1.54 | 13 | 1.48 | 10 | 0.94 | 2 | 0.04 |
| metabolism, lipid | 14 | 1.07 | 19 | 1.14 | 18 | 1.19 | 14 | 1.33 | 8 | 0.57 |
| metabolism, nucleic acid | 7 | 2.48 | 18 | 1.99 | 9 | 0.97 | 9 | 2.19 | 13 | 1.22 |
| mucin | 3 | 0.21 | 5 | 0.53 | 4 | 0.54 | 6 | 0.96 | 4 | 0.27 |
| nuclear regulation | 7 | 0.39 | 7 | 0.45 | 8 | 1.04 | 7 | 1.24 | 7 | 1.00 |
| protease | 17 | 0.72 | 25 | 1.07 | 20 | 0.97 | 25 | 1.46 | 27 | 4.08 |
| protease inhibitor | 55 | 22.28 | 76 | 21.14 | 52 | 11.10 | 64 | 15.33 | 66 | 20.32 |
| proteasome machinery | 8 | 1.57 | 6 | 0.50 | 6 | 1.29 | 6 | 1.50 | 6 | 1.18 |
| protein modification | 16 | 0.59 | 6 | 0.14 | 16 | 0.84 | 7 | 0.22 | 5 | 0.13 |
| protein synthesis | 6 | 0.16 | 2 | 0.05 | 4 | 0.14 | 2 | 0.06 | 0 | 0.00 |
| signal transduction | 14 | 3.57 | 11 | 4.74 | 3 | 0.39 | 7 | 2.01 | 8 | 1.80 |
| tick specific proteins | 110 | 23.99 | 188 | 39.44 | 118 | 37.88 | 140 | 37.87 | 105 | 38.28 |
| transcription machinery | 4 | 0.24 | 1 | 0.03 | 3 | 0.18 | 3 | 0.11 | 0 | 0.00 |
| transporter/ receptor | 10 | 1.12 | 6 | 0.42 | 4 | 0.37 | 7 | 0.62 | 3 | 0.19 |
| transposon element | 1 | 0.02 | 2 | 0.03 | 1 | 0.05 | 0 | 0.00 | 0 | 0.00 |
| Total | 450 | 100.00 | 540 | 100.00 | 419 | 100.00 | 441 | 100.00 | 332 | 100.00 |
Table 2. Numbers and cumulative relative abundance of tick protein classes in Amblyomma americanum saliva during 144 to completion of feeding (NSAF = normalized spectral abundance factor).
| Feeding Time Point | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 144 h | 168 h | 192 h | BD | SD | ||||||
| Classification | Protein Count | NSAF (%) | Protein Count | NSAF (%) | Protein Count | NSAF (%) | Protein Count | NSAF (%) | Protein Count | NSAF (%) |
| antimicrobial | 4 | 4.31 | 4 | 2.87 | 3 | 2.98 | 3 | 5.04 | 3 | 1.49 |
| cytoskeletal | 38 | 3.43 | 11 | 0.98 | 16 | 1.23 | 3 | 0.39 | 14 | 1.42 |
| detoxification | 21 | 1.71 | 11 | 0.67 | 18 | 1.13 | 7 | 0.79 | 12 | 0.55 |
| evasin | 5 | 1.54 | 7 | 3.40 | 6 | 3.39 | 1 | 0.83 | 5 | 2.86 |
| extracellular matrix | 18 | 1.61 | 7 | 0.38 | 19 | 0.85 | 14 | 1.75 | 0 | 0.00 |
| glycine rich | 14 | 1.03 | 19 | 2.00 | 31 | 2.79 | 9 | 0.47 | 8 | 0.56 |
| heme/iron binding | 17 | 22.96 | 14 | 7.31 | 16 | 12.38 | 17 | 26.07 | 10 | 7.23 |
| immune-related | 14 | 3.76 | 9 | 2.37 | 14 | 3.09 | 12 | 4.65 | 7 | 1.66 |
| ixodegrin | 1 | 0.58 | 1 | 0.20 | 1 | 0.62 | 2 | 0.78 | 0 | 0.00 |
| lipocalin | 8 | 1.11 | 24 | 6.57 | 20 | 5.69 | 20 | 4.07 | 26 | 14.76 |
| metabolism, amino acid | 4 | 0.07 | 2 | 0.07 | 1 | 0.04 | 0 | 0.00 | 0 | 0.00 |
| metabolism, carbohydrate | 22 | 1.30 | 4 | 0.12 | 14 | 0.90 | 14 | 2.56 | 5 | 0.17 |
| metabolism, energy | 13 | 0.81 | 9 | 0.34 | 10 | 0.50 | 2 | 0.30 | 6 | 0.15 |
| metabolism, lipid | 16 | 0.95 | 5 | 0.39 | 16 | 1.01 | 12 | 1.29 | 3 | 0.39 |
| metabolism, nucleic acid | 14 | 1.80 | 10 | 0.93 | 16 | 1.72 | 3 | 1.11 | 8 | 1.21 |
| mucin | 7 | 0.33 | 3 | 0.13 | 9 | 0.33 | 3 | 0.56 | 0 | 0.00 |
| nuclear regulation | 10 | 0.74 | 8 | 1.49 | 8 | 1.02 | 1 | 0.26 | 11 | 2.15 |
| protease | 40 | 3.37 | 38 | 4.57 | 43 | 4.21 | 36 | 2.93 | 36 | 7.70 |
| protease inhibitor | 83 | 17.41 | 81 | 15.82 | 78 | 14.79 | 59 | 19.17 | 39 | 17.08 |
| proteasome machinery | 7 | 0.87 | 6 | 1.09 | 6 | 0.87 | 4 | 0.11 | 6 | 1.68 |
| protein modification | 18 | 0.58 | 10 | 0.26 | 13 | 0.38 | 0 | 0.00 | 11 | 0.44 |
| protein synthesis | 2 | 0.03 | 2 | 0.06 | 5 | 0.15 | 0 | 0.00 | 2 | 0.08 |
| signal transduction | 14 | 3.11 | 10 | 1.49 | 9 | 2.43 | 7 | 1.89 | 5 | 0.53 |
| tick specific proteins | 127 | 25.15 | 175 | 46.18 | 157 | 36.86 | 77 | 23.84 | 103 | 37.75 |
| transcription machinery | 2 | 0.04 | 1 | 0.02 | 1 | 0.01 | 0 | 0.00 | 1 | 0.02 |
| transporter/ receptor | 10 | 1.38 | 6 | 0.26 | 6 | 0.62 | 6 | 1.14 | 3 | 0.07 |
| transposon element | 0 | 0.00 | 1 | 0.04 | 0 | 0.00 | 0 | 0.00 | 1 | 0.02 |
| Total | 529 | 100.00 | 478 | 100.00 | 536 | 100.00 | 312 | 100.00 | 325 | 100.00 |
Table 3. Numbers and cumulative relative abundance of rabbit protein classes in Amblyomma americanum saliva during 24–120 h of feeding (NSAF = normalized spectral abundance factor).
| Feeding Time Point | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 96 h | 120 h | ||||||
| Classification | Protein Count | NSAF (%) | Protein Count | NSAF (%) | Protein Count | NSAF (%) | Protein Count | NSAF (%) | Protein Count | NSAF (%) |
| antimicrobial | 3 | 0.00134357 | 4 | 0.00192864 | 3 | 0.00171397 | 5 | 0.0086771 | 6 | 0.00763598 |
| antioxidant | 2 | 0.00042155 | 1 | 7.576E-05 | 1 | 0.00035322 | 0 | 0 | 0 | 0 |
| cytoskeletal | 19 | 0.01798265 | 14 | 0.01629188 | 11 | 0.00953933 | 12 | 0.01698913 | 7 | 0.01026072 |
| extracellular matrix | 3 | 0.00049492 | 1 | 0.000267 | 1 | 0.0000291 | 2 | 0.0001466 | 2 | 0.0003605 |
| fibrinogen | 3 | 0.00022576 | 4 | 0.00017475 | 0 | 0 | 1 | 6.7612E-05 | 2 | 0.00022745 |
| globin/ RBC | 20 | 0.08991802 | 18 | 0.02390767 | 12 | 0.01107268 | 15 | 0.02658361 | 15 | 0.02722383 |
| heme/Iron binding | 5 | 0.01948816 | 7 | 0.01314713 | 2 | 0.00443612 | 7 | 0.00916951 | 4 | 0.00596773 |
| immunity related | 9 | 0.00268497 | 7 | 0.0023492 | 5 | 0.00211006 | 8 | 0.00712898 | 15 | 0.01097912 |
| keratin | 17 | 0.00337019 | 32 | 0.01031924 | 35 | 0.02503904 | 41 | 0.04692297 | 27 | 0.03080678 |
| lipocalin | 0 | 0 | 1 | 0.00051272 | 1 | 0.00052663 | 1 | 0.00283525 | 1 | 0.00167895 |
| metabolism, amino acid | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| metabolism, carbohydrates | 5 | 0.00133936 | 4 | 0.00037672 | 5 | 0.00064097 | 5 | 0.00074812 | 3 | 0.00033307 |
| metabolism, energy | 1 | 2.8207E-05 | 0 | 0 | 0 | 0 | 3 | 0.00071594 | 1 | 0.00018482 |
| metabolism, lipid | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.00093604 | 2 | 0.00138574 |
| metabolism, nucleic acids | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| nuclear regulation | 8 | 0.00325694 | 16 | 0.00871704 | 15 | 0.01681692 | 18 | 0.01938734 | 15 | 0.01609353 |
| protease | 0 | 0 | 1 | 0.00013596 | 0 | 0 | 2 | 0.00146001 | 1 | 4.2416E-05 |
| protease Inhibitors | 8 | 0.0025413 | 5 | 0.00116042 | 1 | 0.0001394 | 5 | 0.00178596 | 1 | 0.00071414 |
| protein export | 0 | 0 | 2 | 0.00017604 | 2 | 0.00115523 | 3 | 0.00209215 | 3 | 0.00247782 |
| protein modification | 7 | 0.00102993 | 3 | 0.0003923 | 7 | 0.00221869 | 6 | 0.00095378 | 4 | 0.00051132 |
| protein synthesis | 4 | 0.00135166 | 2 | 0.0001756 | 4 | 0.00122153 | 4 | 0.0006344 | 0 | 0 |
| proteasome machinery | 2 | 0.00600273 | 2 | 0.0021349 | 2 | 0.00578655 | 2 | 0.0056957 | 2 | 0.00490593 |
| signal transduction | 9 | 0.00264391 | 1 | 0.00047693 | 7 | 0.00264375 | 1 | 0.00089234 | 1 | 0.00035228 |
| transporter/ receptor | 2 | 0.00013431 | 5 | 0.00049163 | 1 | 3.5648E-05 | 4 | 0.00061835 | 0 | 0 |
| transcription machinery | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 127 | 0.15425811 | 130 | 0.08321153 | 115 | 0.08547884 | 147 | 0.15444091 | 112 | 0.12214214 |
Table 4. Numbers and cumulative relative abundance of rabbit protein classes in Amblyomma americanum saliva during 144 to completion of feeding (NSAF = normalized spectral abundance factor).
| Feeding Time Point | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 144 h | 168 h | 192 h | BD | SD | ||||||
| Classification | Protein Count | NSAF (%) | Protein Count | NSAF (%) | Protein Count | NSAF (%) | Protein Count | NSAF (%) | Protein Count | NSAF (%) |
| antimicrobial | 8 | 0.007565 | 9 | 0.018337 | 9 | 0.015212 | 6 | 0.004394 | 8 | 0.015088 |
| antioxidant | 3 | 0.000677 | 2 | 0.00071 | 3 | 0.000384 | 0 | 0 | 4 | 0.000927 |
| cytoskeletal | 20 | 0.019936 | 29 | 0.014844 | 26 | 0.019163 | 6 | 0.011922 | 55 | 0.031601 |
| extracellular matrix | 1 | 3.63E-05 | 0 | 0 | 1 | 2.58E-05 | 1 | 8.41E-05 | 3 | 0.000973 |
| fibrinogen | 1 | 0.00011 | 5 | 0.002512 | 5 | 0.001638 | 0 | 0 | 5 | 0.001423 |
| globin/ RBC | 18 | 0.105112 | 19 | 0.100601 | 18 | 0.067832 | 16 | 0.052725 | 21 | 0.109247 |
| heme/Iron binding | 3 | 0.005258 | 9 | 0.011255 | 7 | 0.011786 | 3 | 0.005854 | 9 | 0.015765 |
| immunity related | 15 | 0.007953 | 23 | 0.02019 | 17 | 0.014264 | 5 | 0.002246 | 23 | 0.025243 |
| keratin | 20 | 0.010315 | 34 | 0.012893 | 37 | 0.021782 | 21 | 0.010099 | 40 | 0.045599 |
| lipocalin | 1 | 0.002122 | 1 | 0.004206 | 1 | 0.00311 | 1 | 0.001514 | 1 | 0.00534 |
| metabolism, amino acid | 0 | 0 | 1 | 0.000201 | 0 | 0 | 0 | 0 | 1 | 3.83E-05 |
| metabolism, carbohydrates | 5 | 0.001337 | 9 | 0.001486 | 7 | 0.001096 | 2 | 0.000114 | 7 | 0.001634 |
| metabolism, energy | 2 | 0.000319 | 2 | 0.000144 | 2 | 0.000364 | 0 | 0 | 6 | 0.000535 |
| metabolism, lipid | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.000229 |
| metabolism, nucleic acids | 1 | 0.000108 | 4 | 0.000989 | 0 | 0 | 0 | 0 | 0 | 0 |
| nuclear regulation | 19 | 0.013472 | 20 | 0.028935 | 24 | 0.022492 | 10 | 0.008565 | 26 | 0.032846 |
| protease | 0 | 0 | 4 | 0.000876 | 1 | 9.16E-05 | 0 | 0 | 6 | 0.00066 |
| protease Inhibitors | 1 | 0.000202 | 3 | 0.000967 | 2 | 0.000151 | 0 | 0 | 7 | 0.00203 |
| protein export | 3 | 0.001588 | 4 | 0.004882 | 5 | 0.003771 | 2 | 0.00077 | 4 | 0.006886 |
| protein modification | 6 | 0.000988 | 7 | 0.000754 | 12 | 0.001567 | 0 | 0 | 16 | 0.002522 |
| protein synthesis | 0 | 0 | 4 | 0.00057 | 4 | 0.00084 | 0 | 0 | 4 | 0.000866 |
| proteasome machinery | 2 | 0.003209 | 2 | 0.003649 | 2 | 0.003418 | 2 | 0.000584 | 2 | 0.005294 |
| signal transduction | 8 | 0.003392 | 5 | 0.001237 | 9 | 0.001945 | 0 | 0 | 12 | 0.003857 |
| transporter/ receptor | 3 | 0.000381 | 1 | 6.81E-05 | 4 | 0.000521 | 3 | 0.000589 | 13 | 0.00182 |
| transcription machinery | 0 | 0 | 2 | 6.16E-05 | 2 | 6.68E-05 | 0 | 0 | 7 | 0.000636 |
| Total | 140 | 0.184079 | 199 | 0.230369 | 198 | 0.191521 | 78 | 0.099459 | 282 | 0.311061 |
Overall, majority of the identified proteins are tick specific proteins (did not match to proteins of non-tick organisms) of unknown function (32%), followed by protease inhibitors (PI) (13%), proteases (8%), and glycine-rich proteins (6%). Notable protein categories that were ≤ 5% include cytoskeletal, lipocalin, antioxidant/detoxification, extracellular matrix, immune related, heme/iron-binding, mucins, evasins, antimicrobials, and ixodegrins (Tables 1 and 2, S1 Table). For rabbit proteins, the majority are categorized as cytoskeletal (19%), followed by keratin (13%), nuclear regulation (8%), immunity-related (8%), globin/RBC degradation (6%), and protein categories that were ≤ 5% include antimicrobials, heme/iron-binding, protease inhibitors, proteases, extracellular matrix, antioxidant/detoxification, fibrinogen and lipocalin (Tables 3 and 4, S1 Table).
The most abundant category of A. americanum tick saliva proteins is tick-specific proteins of unknown function
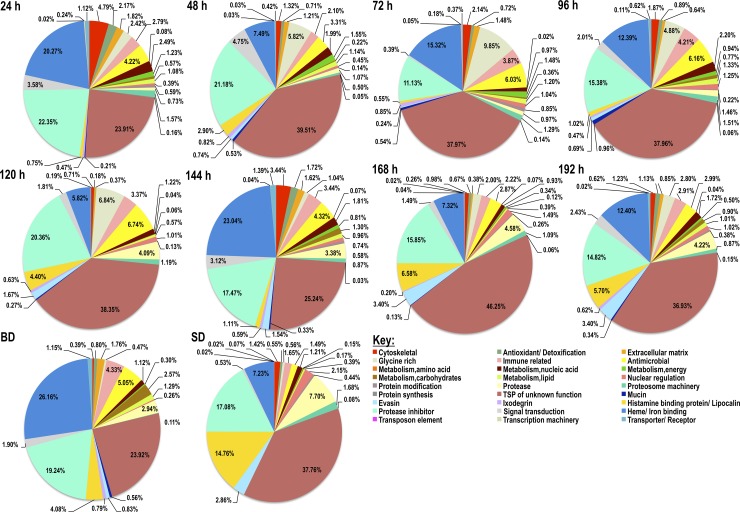
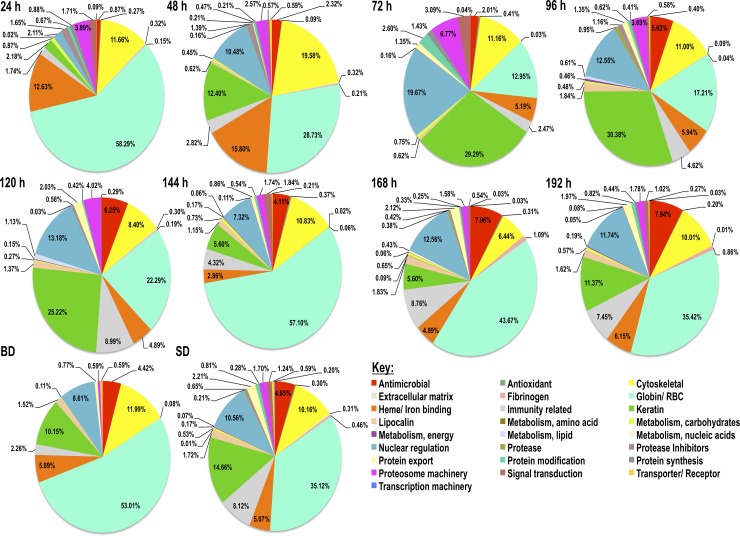
To get insights into relative abundance of each functional category, sum totals of NSAF values for each functional category are summarized in Figs 1 and 2. As shown in Fig 1, tick-specific secreted saliva proteins of unknown function (TSP), protease inhibitors (PI), and heme/iron binding proteins are the most abundant ranging from a respective 24–46%, 11–22%, and 6–26% during feeding (24-192h). Other protein categories at ≤ 10% in abundance include glycine-rich proteins, antimicrobial peptides, evasins, and proteases. For rabbit proteins in A. americanum tick saliva, the most predominant functional category was hemoglobin/red blood cell products (RBC) (13–58%) followed by cytoskeletal (6–20%), heme/iron binding (5–16%), keratin (2–30%), and nuclear regulation (2–20%) (Fig 2). It is notable that rabbit functional categories related to immunity, antimicrobial peptides, protease inhibitors and proteases were abundant at ≤8% throughout feeding.
Fig 1. Tick saliva proteins composition in Amblyomma americanum tick saliva every 24 h during feeding.
Pilocarpine induced A. americanum tick saliva was subjected to LC-MS/MS sequencing using the “In-Solution” digestion approach as described in materials and methods. Cumulative normalized spectral abundance factors (NSAF) values as index for relative abundance of detected tick saliva proteins are presented for each functional class. Please note the labels are oriented horizontally and read left to right starting from cytoskeletal proteins (red), antioxidant/detoxification (green), extracellular matrix (orange), then back to glycine rich proteins (light-tan), immune related (pink) and continues this pattern.
Fig 2. Rabbit host proteins composition in Amblyomma americanum tick saliva every 24 h during feeding.
Pilocarpine induced A. americanum tick saliva was subjected to LC-MS/MS sequencing using the “In-Solution” digestion approach as described in materials and methods. Cumulative normalized spectral abundance factors (NSAF) values as index for relative abundance of detected rabbit host proteins are presented for each functional class. Please note the labels are oriented horizontally and read left to right starting from antimicrobial proteins (red), antioxidant/detoxification (green), cytoskeletal (yellow), then back to globin/RBC (light-green), fibrinogen (pink) and continues this pattern.
The finding that the majority of proteins identified in this study are of unknown function is not unique to A. americanum tick saliva, it is consistent with findings in saliva of I. scapularis [32] and tick salivary gland transcriptomics [59–61]. This is potentially a reflection of how little information exists on the molecular basis of tick feeding physiology.
Majority of A. americanum tick saliva proteins are associated with early stage tick-feeding processes
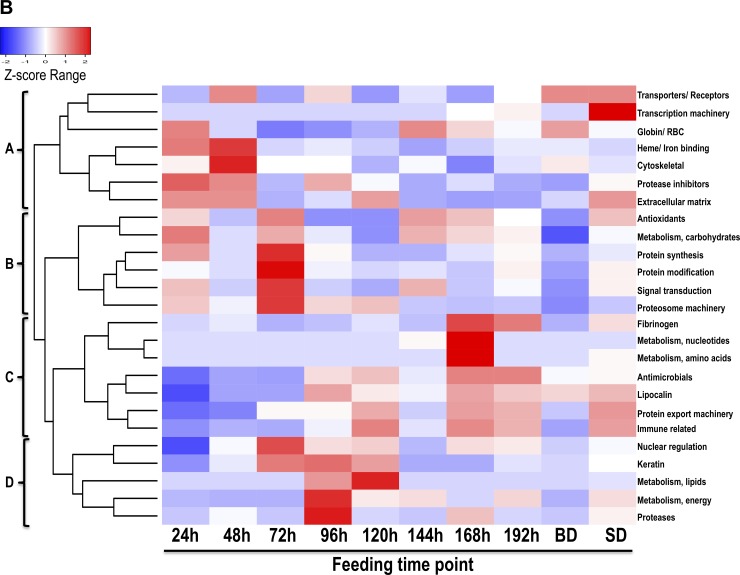
To gain insight into broad relationships of secretion dynamics of both tick and rabbit proteins with the tick feeding processes, Z-score statistics normalized NSAF values were visualized on heat maps (Figs 3 and 4). The clustering patterns are influenced by relationships between secretion dynamics of protein category. The blue to red transition denotes low to high abundance. As shown in Fig 3, the 27 tick protein functional categories clustered into four broad secretion patterns (clusters A-D). It is interesting to note that, 74% (20/27) of functional categories are secreted at high abundance within the first 48 h of feeding (Fig 3, clusters A, B, and C) with exception of four categories (evasins, proteases, lipocalins, and nuclear regulation proteins) in cluster D, which are injected into the host at high abundance starting from day five of feeding.
Fig 3. Overall secretion dynamics of tick saliva protein classes in Amblyomma americanum tick saliva.
Normalized spectral abundance factors (NSAF) values of tick was normalized using the z-score statistics and then used to generate heat maps using heatmap2 function in gplots library using R as described in materials and methods.
Fig 4. Overall secretion dynamics of rabbit host protein classes in Amblyomma americanum tick saliva.
Normalized spectral abundance factors (NSAF) values of rabbit host was normalized using the z-score statistics and then used to generate heat maps using heatmap2 function in gplots library using R as described in materials and methods.
Similarly, the majority of rabbit protein functional categories (21 of the 25) were detected at high abundance in saliva of A. americanum ticks during feeding (Fig 4). The 25 rabbit protein categories in saliva of A. americanum ticks segregated into four clusters, A-D (Fig 4). Rabbit proteins that were secreted at high abundance starting from 24–72 h of feeding are part of clusters A and B. Five of the seven proteins in cluster A are highly abundant at 24 and 48 h feeding time points, while those in clusters C and D were less abundant in the first 48 h of feeding and showed varied abundance levels starting from 72 h of feeding.
Ticks initiate the feeding process by secreting an adhesive substance to anchor onto host skin and creating the feeding lesion by lacerating host tissue within the 24–36 h of attachment [62] followed by transmission of some TBD agents after the tick has attached for more than 48 h [63, 64]. On this basis, we speculate that tick proteins in clusters A-C are related with regulating early stages of tick feeding activities associated with initiating tick feeding and regulating transmission of TBD agents. We also speculate that protein categories that were identified in abundance starting from the 192 h feeding time point might be associated with regulating the end of the tick-feeding process, when the tick detaches from the host skin without causing significant damage to host skin.
Secretion dynamics of non-housekeeping-like A. americanum tick saliva proteins
S1 Table lists individual proteins that were identified in A. americanum tick saliva. Thirteen functional categories not considered as housekeeping-like (antimicrobial, detoxification extracellular matrix/cell adhesion, evasin, glycine-rich, heme/iron binding, immunity-related, ixodegrin, lipocalin, mucin, protease inhibitors, proteases, and TSPs of unknown function) (Tables 1 and 2) accounted for 76% of total number of proteins and represented more than 82% in relative abundance throughout feeding time points. In the subsequent sections, we have discussed non-housekeeping-like tick proteins individually per functional category (S1A–S1S Fig) and have highlighted housekeeping-like tick proteins and rabbit proteins as a group below. We have presented and discussed data in S1 Fig. based on similarities in secretion patterns. Please note that the dendrograms were hierarchically clustered based on abundance throughout feeding (using gplot2 software in R). We then manually labeled (with letters) the clusters to discuss the secretion dynamic profiles. Our group is interested and is working to understand functions of proteases and protease inhibitors, and our subsequent discussion below is biased toward these two categories.
a) A. americanum tick saliva contains a large diversity of protease inhibitors in nine families
We previously documented at least 18 of the 99 Merops database protease inhibitors (PI) that might be expressed by A. americanum and other tick species [65]. Here we show that adult A. americanum ticks secreted at least 155 PIs belonging into eight Merops PI families (S1A–S1E Fig). These include Kunitz-type inhibitors (I2, n = 68), serine protease inhibitors (serpins, I4, n = 21), trypsin inhibitor-like (TIL, I8, n = 36), alpha-2-macroglobulins (α2M, I39, n = 12), cysteine inhibitors (cystatin, I25, n = 12), thyropins (I31, n = 3), phosphatidylethanolamine-binding proteins (I51, n = 2), and a tick carboxypeptidase inhibitor (TCI, n = 1). Of significant interest, nearly 75% of PIs (115/155) in this study were secreted in saliva within the first 120 h of feeding (S1 Table). This strongly suggests that functions of tick saliva PIs are associated with regulating early stages of tick feeding that include tick transmission of TBD agents.
Of the PI families in this study, serpins are the most studied [42, 65–70], presumably because functional roles of this protein category are relatable to tick feeding physiology. To successfully feed and transmit TBD agents, ticks have to overcome serine protease-mediated host defense pathways that are tightly controlled by inhibitors, including serpins. On this basis, it was proposed that ticks might utilize serpins to evade host defenses to successfully feed [71]. From this perspective, it is notable that 90% (19/21) of serpins were identified in saliva of ticks that fed for 24–48 h (S1A Fig), suggesting these serpins are injected into host and might be involved with regulating tick feeding within hours of the tick starting to feed. We would like to note that 5 of the 19 serpins that were identified in saliva of 24–48 h of attachment (Aam-264383, Aam-88534, Aam-264380, Aam-433905, and Aam-1014495) were secreted at high abundance in later feeding time points. It is interesting to note that, A. americanum serpin 6 (Aam-2673) and 19 (Aam-88534), which were previously validated as inhibitors of host defense system proteases including blood clotting [72, 73] were also found in tick saliva within the first 24 h of feeding this study. Most recently, AAS27 (Aam-28434), that has anti-inflammatory function by inhibiting trypsin and trypsin-like proteases were present in all time points in tick saliva [74]. In another study, we have shown that, AAS41 (Aam-973854), which is secreted at high abundance continuously through 120 h is an inhibitor of inflammation that acts by inhibiting chymase (Kim et al., in submission). It is also notable that whereas, 20–50% of all PIs were identified at a single time points, only a single serpin (Aam-264383) was found at a single time point (S1 Table). This might suggest that the functions of tick saliva serpins are important throughout the tick feeding process.
Although not much has been reported on the functional analysis of A. americanum tick cystatins, a lone study has reported that RNAi-mediated silencing of a cystatin transcript reduced the ability of ticks to feed successfully [75]. Several researchers have reported cystatins in other tick species indicated that they play important roles in tick feeding physiology [76]. In the soft tick Ornithodoros moubata, a cystatin was internalized by host dendritic cells and targeted cathepsin S and cathepsin C, affecting their maturation [77]. Cystatins from other tick species also have immunosuppressive functions [78–80]. Of the 12 cystatins identified in tick saliva from this study (S1B Fig, S1 Table), seven were secreted on or after 72 h of feeding, indicating that majority of cystatins might be involved in regulating tick feeding functions after the tick has initiated feeding. On the contrary, five cystatins were secreted within the first 48 h of feeding.
Similar to cystatins, S1C Fig shows the secretion dynamics of alpha-2-macroglobulins (α2M), where less than half (4/12) were injected into the host at high abundance at start of feeding (24–48 h), and another two at the 96 h feeding time point (clusters A and B). It is also notable that the remaining 6 of 12 were injected at high abundance at the 144 h time point (clusters C and D). This might suggest that α2M could be involved in regulating tick feeding functions toward the end; we normally observe ticks starting to engorge and complete feeding after 120 h of attachment. There are very few studies on α2M in tick feeding physiology. Two studies have reported involvement of α2M in immune defense of soft ticks [81] and anti-microbial activity in I. ricinus [82].
Kunitz-type inhibitors and trypsin inhibitor-like (TIL) were the majority of PIs that were found in this study. The secretion dynamics of Kunitz-type inhibitors (S1D Fig) and TIL (S1E Fig) is comparable and notable: the tick appears to secrete a different set of these inhibitors every 24 h starting from the first day of tick feeding. This might suggest that functions of these inhibitors are required throughout the tick feeding process. The observed alternate secretion pattern might signal the potential for ticks to evade host defense in that host immunity against 24 h secreted inhibitors might not be effective against a different set that is secreted at subsequent time points. It is also notable that a total 35% of Kunitz-type inhibitors and 22% of TILs were detected in saliva of replete fed ticks (SD), unlike the other PIs in this study, suggesting a role toward end of tick feeding.
b) Majority of proteases in A. americanum saliva are metalloproteases
At the time of this study, protease families that were encoded by A. americanum were not enumerated, presumably because its genome has not been sequenced. However, analysis of annotated sequences from I. scapularis showed that the tick might encode for all protease categories: aspartic, cysteine, serine, metallo-, and threonine proteases [83]. Here, we found that A. americanum secretes at least 94 proteases in saliva during feeding. These 94 proteases belong in four categories grouped into 15 families: aspartic (family A1, n = 4), cysteine (C1, C2, and C13, n = 12), metallo- (M12, M13, M14, M15, M17, M20, M28, and M49, n = 56), and serine (S1, S10, and S28, n = 22) proteases (S1 Table, S1F, S1G and S1H Fig). Please note that the heatmap for aspartic proteases was not developed due to low numbers (the secretion dynamics is presented in S1 Table). The heatmaps (S1F, S1G and S1H Fig and S1 Table) show that more than 60% (60/94) of proteases are injected into the host at various time points during the first five days of feeding, demonstrating that some of the proteases in this study are associated with regulation of initial tick feeding processes.
The observation that metalloproteases are the majority of proteases in saliva of A. americanum is consistent with our previous findings in the I. scapularis proteome [32]. It is notable that similar to the I. scapularis proteome, metalloproteases that were secreted at high abundance during the first 72 h feeding time points are in families M12 and M13 (S1G Fig), indicating that these proteases regulate initial tick feeding functions that are important to both tick species. Indirect evidence on snake venom M12 proteases that have anti-coagulant activity [84, 85] suggest that secretion of these proteases at high abundance when the tick is initiating feeding might be beneficial to the tick as they might function to prevent blood from clotting. There is also evidence that RNAi-mediated silencing of M12 proteases significantly affected tick-feeding efficiency [86]. There is evidence that I. scapularis secretes a metalloprotease that is similar to snake venom hemorrhagic proteases that degrades gelatin, fibrin(ogen), and fibronectin [87]. Likewise, indirect evidence suggests that ticks might utilize M13 proteases to regulate host immunity. In mammals, M13 proteases were among other functions, involved in modulating neurotransmitter levels, control blood pressure, involved in reproduction and cancer progression [88].
Another notable similarity between A. americanum and I. scapularis proteomes is that both tick species secreted a small number of S1 serine proteases, six and three respectively (S1G Fig and S1 Table). We are interested in S1 serine proteases due to their functional roles in signal transduction as activators of protease-activated receptors [89, 90]. Therefore, it is potentially possible that the tick utilizes these proteases to interfere with host defense signaling at the tick-feeding site.
The observation that A. americanum injected cysteine proteases at the beginning of feeding indicate they might be playing some role(s) in the early stages of tick feeding. Several studies have documented potential functional roles of cysteine proteases in tick physiology [91–93]. In a lone study, a cysteine protease from H. longicornis when silenced by RNAi, showed to be involved with digestion of a blood meal and increased the number of Babesia parasites [94]. Recently, a cathepsin L from the tick, R. microplus (BmCL1), was shown to interact with thrombin at pH 7.5 and impair thrombin-induced fibrinogen clotting via a fibrinogenolytic activity [95]. In helminths, cysteine proteases are the most abundant category of proteins identified into excretion/secretion products [96] and have been shown to be involved with host immune evasion [97] and extracellular matrix degradation [98].
Majority of studies on tick aspartic proteases are mainly characterized as blood digestion proteins in the midgut, similar to the mammalian lysosome acidic protease, cathepsin D [99]. In H. longicornis adult ticks, the potential role of these proteins in proteolysis of erythrocyte hemoglobin has been reported [100]. Other studies have shown the importance of this protease in embryogenesis, playing roles in vitellin degradation [101] and heme-binding properties [102]. Although only four aspartic proteases were identified in A. americanum saliva during feeding, three of these proteases were present within the first 96 h of feeding, which may implicate roles in the early stages of tick feeding success (S1 Table).
c) Lipocalins/histamine-binding proteins are alternately secreted during tick feeding
Inflammation response is among host defense pathways that ticks must evade to complete feeding. Histamine is one of the key mediators of inflammation in tissue damage that is expected to occur in response to tick feeding [103]. From this perspective, lipocalins/tick histamine-binding proteins in tick saliva are suspected to be part of the tick machinery to evade the host’s inflammation defense response through sequestration of histamine that is released at the tick-feeding site. In this study, we found 46 lipocalins/tick histamine-binding proteins that show two broad secretion patterns: secreted at multiple feeding time points and those that were alternately secreted at single time points (S1I Fig). It is interesting to note that of the total 46 lipocalins identified in tick saliva during feeding, 22% (10/46) were present within the first 48 h of feeding, while 35% (16/46) were present after 96 h of feeding, and 43% (20/46) were identified in a single time point (S1I Fig, S1 Table). Given that in addition to regulating inflammation, lipocalins/histamine-binding proteins have other diverse functions such as antimicrobials [104, 105], glucose metabolism [106] and binding several ligands including serotonin and fatty acids [107, 108], it is most likely that these proteins might be involved in regulating several other tick feeding functions besides mediating the tick’s anti-inflammation function.
d) Heme binding proteins are secreted at high abundance throughout feeding
Like other animals, ticks require iron and heme (the iron-containing part of hemoglobin) for normal physiological functions [109]. However, ticks do not have a heme biosynthesis pathway, therefore they must obtain it from host blood [110]. Female ticks that were artificially fed a diet not containing hemoglobin laid sterile eggs [111] demonstrating the importance of heme in tick biology. However, in high abundance heme can be toxic for the tick [112], therefore it is postulated that hemelipoproteins and vitellogenenins could serve as heme binding proteins to remove the excess heme from the tick system. S1J Fig and S1 Table lists a total of 17 heme/iron-binding proteins consisted of hemelipoproteins, vitellogenins, and a ferritin that collectively accounted for the third most abundant protein category in tick saliva throughout feeding. High abundance of hemelipoproteins here is in consistent with other tick saliva proteomes [32–34]. The secretion dynamics summarized in S1J Fig revealed two broad secretion patterns, those that are injected into the host from 24 h through 120 h of feeding (HCB and HCC) and those that are injected into the host starting from 144 h of feeding through the end of tick feeding (HCD and HCA). Ticks acquire both iron and heme from host blood [110, 113], and thus iron and/heme-binding proteins are important to normal tick physiology. It has been shown that R. microplus hemelipoprotein (HeLp) could bind eight heme molecules [114]. Given that hemelipoprotein is the most abundant protein in tick hemolymph [115], it could be secreted in saliva as a result of this protein being transferred into the salivary glands. There is also evidence that mRNA of hemelipoproteins is also expressed in salivary glands [116] of unfed and fed adult ticks and might suggest that these proteins could have different functions during blood meal feeding by ticks. It is also known that free heme has pro-inflammatory properties [117]. Thus, the presence of hemelipoproteins could lower free heme concentration at the feeding site, and as a result reduce the potential for heme to promote inflammation. Indirect evidence suggest that tick saliva hemelipoproteins might also serve as antioxidants and transporters of other compounds such as cholesterol, phospholipids, and free fatty acids [118]. The role of vitellogenin-like proteins in tick feeding remain to be established. It is interesting to note that although vitellogenin is predominant, reduction of vitellogenin receptor (VgR) expression by RNAi-mediated silencing resulted in reduced fertility [119] and Babesia bovis transmission and oocyte maturation [120].
e) Ticks inject multiple antioxidant proteins into the feeding site
Feeding and digestion of large amounts of host blood exposes ticks to hydroxyl radicals and reactive oxygen species (ROS), which if left uncontrolled could damage tick tissue [121, 122]. Expression of antioxidant proteins protect the tick during feeding and digestion of the blood meal. Studies have shown that RNAi-mediated silencing of tick antioxidants caused deleterious effects to the tick and prevented them to obtain a full blood meal [123, 124]. Previous studies by others and from our group have documented presence of antioxidants in tick salivary glands [125, 126] and saliva [32–34, 127, 128]. In this study we identified 41 putative antioxidant enzymes. These enzymes include glutathione-S-transferase, thioredoxin, superoxide dismutase, catalase, peroxinectin, arylsulfatase, aldehyde dehydrogenase, epoxide hydrolase, sulfotransferase, sulfhydryl oxidase and glycolate oxidase. S1K Fig reveals two broad secretion patterns of tick saliva antioxidant proteins based on NSAF values as an index for abundance: (i) proteins injected into the host in high abundance once at various feeding time points (ACA-ACI) and (ii) proteins that are consecutively injected into the host in high abundance starting from 24–96 h of feeding (ACF). Like heme/iron binding proteins, tick antioxidants are presumed to function inside the tick and the role(s) these proteins in the feeding regulation remain to be defined. We speculate that as host tissue injury caused during the creation of the tick-feeding site could trigger release of oxidants such as ROS, some of the tick saliva antioxidants might function to counter oxidant molecules to protect tick tissue.
f) Glycine-rich and extracellular matrix/cell adhesion proteins are secreted early during tick feeding
Within 5–30 min of attachment, the tick secretes an adhesive substance called cement, which anchors ticks onto host skin during its protracted feeding period [62]. Tick cement is also suggested to protect the tick from host immune factors [129, 130] and might function as antimicrobials at the feeding site [131]. Glycine-rich proteins are among categories of tick proteins that are thought to play key roles in formation of tick cement [62]. From this perspective, glycine-rich proteins are among tick proteins that have received significant research attention [132–135]. In this study we found a total of 67 glycine-rich proteins, which represented the fifth largest category of proteins identified in tick saliva during feeding (Fig 1, S1 Table). Nearly 90% (60/67) of the glycine-rich proteins were secreted in abundance within the first four days of feeding (S1L Fig; GCB, GCD, GCE, GCF, and GCG). Tick cement deposition is completed during the first 96 h of tick feeding [62], and thus it is conceivable that some of the glycine-rich proteins in this study might be involved with tick cement formation. It is interesting to note that some of the glycine-rich proteins that were identified from tick cement in our group [134] and others [133] were also found in this study (S2 Table). Some of the glycine-rich proteins were secreted from the 144 h time point, long after tick cement formation; these might regulate other tick feeding functions. Although glycine-rich proteins are mostly known for their potential role in tick cement formation, indirect evidence in other organisms indicate that these proteins might be involved in other functions such as host defense and stress response as in plants [136].
S1M Fig. summarize the secretion dynamics of 37 extracellular matrix proteins that were found in this study. Similar to glycine-rich proteins, majority (27/37) of the extracellular proteins were secreted within the first five days of feeding demonstrating their role in early stage tick feeding regulation. Our speculation is that some of these proteins will play roles in formation of tick cement. In a previous study, RNAi-mediated silencing of chitinase, also identified in this study, weakened the tick cement cone to the extent that host blood was leaking out around the mouthparts of attached ticks [40].
g) Antimicrobials, mucins, and immune related proteins are secreted throughout the feeding process
Once the tick has anchored itself onto the host skin and created its feeding lesion, it faces a difficult task of overcoming host humoral and cellular immunity, and also preventing microbes in the host skin from colonizing the tick-feeding site. Here we show that A. americanum secretes immunomodulatory and antimicrobial peptides starting within the early stages of the tick feeding process (S1N–S1R Fig). We identified nine antimicrobials consisting of microplusins, lysozymes, and defensins (S1N Fig). Previous studies showed that microplusin has dual effects against fungus and gram-positive bacteria, lysozyme against gram-positive bacteria, and defensin effective against both gram-positive and -negative bacteria [137–139]. The heat map in S1N Fig shows that antimicrobials were injected into the host starting at 24 and 48 h (AMCA), from 72 h (AMCC), and from 120 h (AMCB). This secretion pattern suggests that the functions of antimicrobial peptides are needed throughout feeding.
Similar to antimicrobials, we identified 12 mucins (S1O Fig), with ~60% of these proteins (7/12) being secreted at high abundance within 24–48 h of feeding. Functional roles of mucins in ticks have not been studied. However, indirect evidence in mammals suggest that mucins might be involved in antimicrobial activity in that human mucins were shown to encapsulate microbes [140].
Among putative immunomodulatory proteins, we identified evasins (S1P Fig) and ixodegrins (S1Q Fig). Evasins (n = 12, S1P Fig) were shown to bind to chemokines [141, 142] to reduce leukocytes recruitment to the tick feeding site and therefore contribute to tick evasion of the host’s inflammatory defense. It is interesting to note that the 12 evasins identified in tick saliva were present after 24 h of feeding and continued to be secreted throughout feeding at variable levels. This might suggest that evasins might not be involved in regulating tick feeding functions during the first 24 h of tick feeding.
S1Q Fig summarizes the secretion pattern of the six ixodegrin-like proteins found in tick saliva during feeding in this study. It is interesting to note, 83% (5/6) of these proteins were identified within the first 48 h of feeding (S1 Table). These proteins were first described in I. scapularis as inhibitors of platelet aggregation [143]. Platelet aggregation is the first step in the blood clotting system [144], which ticks must overcome to successfully feed. Thus, the presence of ixodegrins in saliva of A. americanum at the start of feeding is beneficial to tick feeding success. Finally, we also found proteins that show similarity to previously characterized immunomodulatory proteins (S1R Fig), which have been validated in other tick species including p36, which inhibits cell proliferation and cytokine expression [145]. These proteins might play roles in mediating the tick’s evasion of host immunity.
h) Tick-specific secreted saliva proteins (TSP) of unknown function are alternately secreted
Over one-third of Ixodidae protein sequences deposited into GenBank are annotated as hypothetical, secreted, conserved and unknown proteins. However, some are annotated based on sequence identities and conserved signature motifs, which include basic tail/tailless proteins, 8.9 kDa protein family, leucine-rich proteins, AV422 (a tick saliva protein that is high upregulated when ticks are stimulated to start feeding [39, 146]), proteins containing RGD motifs, which might play roles in inhibition of platelet aggregation [143, 147]. In this study we have identified a total of 377 (S1S Fig) tick saliva proteins that fit the above description that we refer here to as tick-specific saliva proteins of unknown functions (TSPs). More than 95% (357/377) of the total TSPs were identified within the first eight days of feeding in tick saliva indicating their potential roles in regulating the tick feeding process. It is interesting to note that the secretion pattern for over a third (128/377) of the total TSPs identified in tick saliva during feeding were alternately injected once during feeding (S1 Table). From the perspective of finding target antigens for tick vaccine development, TSPs represent a unique opportunity in that they do not share any homology to host proteins and might not cross-react with the host.
A. americanum secretes multiple housekeeping-like proteins in saliva throughout the feeding process
S1 Table lists 288 housekeeping-like proteins that were identified in this study. Presence of these proteins in A. americanum saliva is not unexpected, as similar findings have been previously reported in tick saliva [32–34]. The 288 housekeeping-like proteins were classified into 14 categories including those associated with metabolism of amino acids (n = 7), carbohydrates (n = 25), energy (n = 31), lipids (n = 31), and nucleic acids (n = 33). Other protein categories include those involved in cytoskeletal (n = 53), nuclear regulation (n = 16), protein modification (n = 21), proteasome machinery (n = 8), protein synthesis (n = 10), signal transduction (n = 24), transposable element (n = 3), transcription machinery (n = 7), and transporter/receptors (n = 17). It is interesting to note that, within the first 24 h of feeding 12 of the 14 categories were identified at high abundance (S1A Fig).
One feature of housekeeping-like tick proteins is that they have high sequence identity with mammalian housekeeping proteins, and for this reason they are discounted as potential target antigens for tick vaccine development. However, based on literature showing that several roles of these proteins in host defense, we think that these proteins play an important role in tick feeding physiology. Housekeeping-like proteins identified here mostly function intracellularly, and they serve as alarm signals to alert the host defense system to injury when secreted outside of the cell [148]. There is evidence that in the extracellular space, some of the housekeeping proteins such as heat shock proteins, have anti-inflammatory functions [149], while histone proteins have antimicrobial activity [150]. Given high sequence similarity to host housekeeping proteins, it is possible that some of the tick housekeeping-like proteins play roles in promoting tick feeding through anti-inflammatory and anti-microbial activity.
Another important aspect of tick feeding physiology that has not received much attention is the fact that host blood meal also contains carbohydrates, lipids and other molecules besides host proteins. It is notable that some of the tick housekeeping-like proteins in tick saliva have high similarity to enzymes in the carbohydrate and lipid metabolism pathways. It will be interesting to determine if injecting these proteins into the feeding site helps the tick to break down host blood carbohydrates and lipids into units that can be easily taken and assimilated by ticks.
Secretion of rabbit host proteins in A. americanum tick saliva is not random
In this study, we identified 335 rabbit host proteins belonging into 25 different functional categories that include cytoskeletal (19%), keratin (13%), nuclear regulation (8%), immune-related (8%), hemoglobin/RBC degradation (6%), transporters/receptors (5%), protein modification (5%), and protein categories below 4% included antimicrobials, extracellular matrix, heme/iron binding, detoxification/ antioxidants, metabolism (energy, carbohydrates, lipid, amino acid, and nucleic acids), protein export, protein synthesis, fibrinogen, protease inhibitors, proteases, signal transduction, transcription machinery, proteasome machinery, and lipocalin (Tables 3 and 4, S1 Table). Relative abundance as determined by NSAF indicated that the most abundant protein categories consisted of hemoglobin/RBC degradation products (58–13%), followed by heme/ iron binding host proteins (13–16%), and cytoskeletal (6–20%) (Fig 4).
At a glance, presence of rabbit host proteins in A. americanum tick saliva could be dismissed as host protein contamination. This observation might be strengthened by the fact that some rabbit host proteins in tick saliva such as keratin, nuclear regulation proteins, and host antimicrobial peptides increased in abundance as feeding progressed. This suggested that secretion of host proteins into tick saliva was a consequence of ticks ingesting an increased amount of host blood, and that some of these host proteins might leak or be regurgitated back into the host via saliva or esophagus. However, our data here suggests that the tick might systematically be utilizing host proteins to regulate its tick-feeding site. For instance, mammals are likely to encode for more than 500 proteases and 150 protease inhibitors (based on rat, mice, and humans [151]), however we found 9 proteases and 8 protease inhibitors from host origin in A. americanum tick saliva (S1 Table). We are of the view that if secretion of host proteins was a random process, we could have identified more rabbit host proteases and protease inhibitors. There are reports that human α1-antitrypsin and α2-macroglobulin are secreted following injury as occurs during tick feeding, and if left uncontrolled could lead to delayed wound healing [152], which is beneficial to tick feeding. On this basis, it is highly likely that ticks inject host α1-antitrypsin and α2-macroglobulin into the feeding site as a strategy of evading the host’s tissue repair defense response. It is also notable that fibrinogen and neutrophil gelantinase-associated lipocalin, which among other functions plays important roles in wound healing, were identified towards the end of feeding [153–156]. This is interesting in that the tick-feeding lesion is completely sealed, preventing leakage of blood, when a replete fed tick detaches from its feeding site. It has been reported in Opisthorchis viverrini, the human liver fluke, that they secrete proteins in the granulin family that accelerate wound healing [157]. It is possible that the increased abundance of host proteins involved in wound healing are secreted by the tick into the feeding site towards the end of tick feeding is the tick’s way to help its host heal.
Different tick species might utilize similar proteins to regulate feeding
At the time of preparing data in this study for publication, several other tick saliva proteomes had been published. We took advantage of the availability of these data to test the hypothesis that key proteins that are important to tick feeding might be conserved across tick taxa. Thus, we compared data in this study to saliva proteomes of I. scapularis [32], R. microplus [33], H. longicornis [34], R. sanguineus [35], D. andersoni [36], and O. moubata [37]. This analysis revealed that more than 24% (284/1182) of the A. americanum tick saliva proteins in this study have homologs that were secreted in saliva of other tick species (S3 Table). Table 5 highlights the 163, 138, 137, 92, 22, and 11 A. americanum tick saliva proteins in 22 functional categories that were >70% identical to proteins in saliva of I. scapularis [32], H. longicornis [34], D. andersoni [36], R. microplus [33], O. moubata [37] and R. sanguineus [35], respectively.
Table 5. Amblyomma americanum tick saliva protein categories that are conserved in other tick saliva proteomes at 70% identity.
| Classification | I. scapularis | H. longicornis | D. andersoni | R. microplus | O. moubata | R. sanguinues |
|---|---|---|---|---|---|---|
| Cytoskeletal | 36 | 27 | 19 | 12 | 3 | 0 |
| Detoxification | 13 | 9 | 9 | 5 | 0 | 2 |
| Extracellular matrix | 3 | 6 | 9 | 3 | 0 | 1 |
| Glycine rich | 5 | 4 | 4 | 4 | 0 | 0 |
| Immune related | 4 | 3 | 3 | 4 | 1 | 1 |
| Metabolism, amino acids | 4 | 1 | 0 | 0 | 0 | 0 |
| Metaolism, carbohydrates | 4 | 3 | 6 | 1 | 1 | 0 |
| Metabolim, energy | 20 | 11 | 12 | 0 | 4 | 0 |
| Metabolism, lipids | 2 | 1 | 3 | 4 | 0 | 0 |
| Metabolism, nucleic acids | 11 | 9 | 1 | 0 | 2 | 0 |
| Nuclear regulation | 6 | 5 | 6 | 4 | 0 | 0 |
| Protein modification | 16 | 14 | 8 | 9 | 7 | 0 |
| Protease | 6 | 6 | 8 | 3 | 0 | 0 |
| Proteosome machinery | 7 | 6 | 5 | 0 | 0 | 6 |
| Protein synthesis | 4 | 2 | 3 | 2 | 0 | 0 |
| Secreted saliva proteins | 5 | 6 | 22 | 14 | 0 | 0 |
| Lipocalin | 0 | 0 | 0 | 4 | 0 | 0 |
| Protease Inhibitors | 5 | 14 | 10 | 11 | 1 | 1 |
| Signal transduction | 6 | 3 | 6 | 1 | 1 | 0 |
| Heme/Iron binding | 1 | 7 | 2 | 9 | 2 | 0 |
| Transcription machinery | 2 | 0 | 1 | 1 | 0 | 0 |
| Transporters/ receptors | 3 | 1 | 0 | 1 | 0 | 0 |
| Total Protein Matches | 163 | 138 | 137 | 92 | 22 | 11 |
Of the 22 categories of proteins, immune-related proteins were present in all tick saliva proteomes. Likewise, proteins from nine other categories (antioxidant/detoxification, carbohydrate metabolism, cytoskeletal, extracellular matrix, heme/iron binding protease, protease inhibitor, protein modification, and signal transduction) from A. americanum saliva were present in saliva of five other tick species. It is notable that tick-specific proteins were the highest conserved among tick species with the exception of R. sanguineus, for which limited data is available. It is notable that five A. americanum TSPs were highly conserved in I. scapularis, a Prostriata tick, and 6, 22, and 14 in Metastriata ticks; H. longicornis, D. andersoni, and R. microplus, respectively.
Of the 22 categories of proteins, immune-related proteins were present in all tick saliva proteomes. Likewise, proteins from nine other categories (antioxidant/detoxification, carbohydrate metabolism, cytoskeletal, extracellular matrix, heme/iron binding protease, protease inhibitor, protein modification, and signal transduction) from A. americanum saliva were present in saliva of five other tick species. It is notable that tick-specific proteins were the highest conserved among tick species with the exception of R. sanguineus, for which limited data is available. It is notable that five A. americanum TSPs were highly conserved in I. scapularis, a Prostriata tick, and 6, 22, and 14 in Metastriata ticks; H. longicornis, D. andersoni, and R. microplus, respectively.
We would like the reader to note that with the exception of I. scapularis tick saliva proteome for which proteins were identified every 24 h during feeding [32] the other tick saliva proteomes were limited to a narrow range of tick feeding time points and/or fully engorged ticks. This might be the reason that higher numbers of tick saliva proteins were conserved between A. americanum and I. scapularis tick saliva proteome. It is interesting to note that, A. americanum and I. scapularis are biologically different as they belong to different tick lineages, Prostriata and Metastriata [158, 159]. Thus, tick saliva proteins that are shared between these two tick species could regulate evolutionarily conserved proteins essential for tick feeding physiology functions. On this basis, such proteins could be targeted for tick vaccine development. We have previously shown that RNAi-mediated silencing of A. americanum tick saliva serpin 19, an anti-coagulant, [73], which is also conserved in I. scapularis ticks [42, 65], caused significant mortality demonstrating the importance of this protein in tick physiology.
Conclusion and future perspective
This study has made a unique contribution toward understanding the molecular basis of A. americanum tick feeding physiology. We believe that this study provides a good starting point toward discovery of effective targets for anti-tick vaccine development. Our strategy to identify tick saliva proteins every 24 h during feeding has allowed us to map tick saliva proteins to different phases of the tick feeding process. This is significant as it provides for the opportunity to focus on tick saliva proteins that regulate the tick feeding process that precede critical events such as TBD agent transmission. Majority of TBD agents are transmitted after 48 h of tick attachment [63, 64], and therefore proteins that are secreted from 24 and 48 h of tick feeding time points are prime candidates for tick vaccine research. It is important to acknowledge the fact that, during the course of feeding, A. americanum ticks secretion of more than 1500 tick and rabbit host proteins might indicate that the tick has inbuilt systems to evade host immunity, and that it is going to be a challenge to actually find effective targets for anti-tick vaccine development. However, the findings that nearly 300 A. americanum tick saliva proteins were also secreted by other tick species is very encouraging as these proteins might provide insight into conserved mechanisms that are utilized by all ticks to successfully feed and could serve as potential targets for anti-tick vaccine development.
We have recently described proteins (n = 340) in saliva of unfed A. americanum ticks that were stimulated to start feeding on three different hosts: rabbits, dogs, and humans [38]. It is notable that 70% (231/340) of proteins in saliva of unfed A. americanum ticks were found in the tick saliva proteome described here (S4 Table). The significance of these data is that the 231 tick saliva proteins present in saliva of both unfed and fed ticks represent proteins that are potentially injected into the host within minutes of the tick attaching onto host skin and are likely associated with regulating initial tick feeding events. Immunologically blocking functions of these proteins might significantly disrupt tick feeding and prevent transmission of TBD agents. In summary, this study has set the foundation for in-depth studies to understand A. americanum tick feeding physiology and find effective targets for development of tick-antigen based vaccines to prevent TBD infections. It is important to note here that, while this study has provided a valuable starting point in discovery of anti-tick vaccine antigens, the next phase of the research to define the anti-tick vaccine efficacy is the most critical.
Supporting information
Normalized spectral abundance factors (NSAF) values of tick saliva proteins that did not show similarity to housekeeping proteins were normalized using the z-score statistics and then used to generate heat maps using heatmap2 function in gplots library using R as described in materials and methods. (Protease Inhibitors are labeled as A- Serpins, B- Cystatins, C- ⍺2-macroglobulin, D-Kunitz type, E- trypsin inhibitor like; Protease are labeled as F- cysteine, G- metalloprotease, H- serine; and other protein classes as I- Lipocalin, J- heme/iron binding, K- antioxidants, L- glycine rich, M- extracellular matrix, N- antimicrobial, O- Mucin/ mucin-like, P- Evasin, Q- Ixodegrin, R- Immune related, and S- tick specific secreted saliva proteins of unknown function).
(TIFF)
The peptide count, spectral count, normalized spectral abundance factor (NSAF), exponentially modified protein abundance index (EMPAI), spectral count, and sequence coverage (%) generated from the Prolucid and IDcompare analyses are represented on an excel file with different tablatures for tick, rabbit host, contaminants, and reversed-sequences. The time point during tick feeding is noted for every 24 h, BD represents manually detached ticks that were apparently fully engorged but not yet detached, and SD represents spontaneously detached fully engorged ticks. The contig represents the identifier for the CDS extracted from the assembled BioProject accession # PRJNA226980. The description is the nomenclature of the putative protein, the classification represents the proteins functional category, occurrence represents the number of times the peptides matching to putative proteins were identified using LC-MS/MS during feeding time points, and status is a binary representation of when the peptides matching to putative proteins were detected (1) or not (0) during feeding time points.
(XLSX)
A. americanum tick saliva proteins identified using LC-MS/MS from this study were compared to currently published A. americanum ticks saliva proteomes. The peptide count, spectral count, normalized spectral abundance factor (NSAF), exponentially modified protein abundance index (EMPAI), spectral count, and sequence coverage (%) generated from the Prolucid and IDcompare analyses are represented on an excel file. The time point during tick feeding is noted for every 24 h, BD represents manually detached ticks that were apparently fully engorged but not yet detached, and SD represents spontaneously detached fully engorged ticks. The identifier represents the contig for the CDS extracted from the assembled BioProject accession # PRJNA226980, or from NCBI GenBank accession numbers. Identifiers with no matches were noted with N/A. The description is the nomenclature of the putative protein, the classification represents the proteins functional category, occurrence represents the number of times the peptides matching to putative proteins were identified using LC-MS/MS during feeding time points, and status is a binary representation of when the peptides matching to putative proteins were detected (1) or not (0) during feeding time points.
(XLSX)
A. americanum tick saliva proteins identified using LC-MS/MS from this study were compared to currently published tick saliva proteomes of I. scapularis, H. longicornis, R. microplus, D. andersoni, O. moubata, and R. sanguineus. Contig numbers are noted for A. mericanum proteins that matched (+) or not matched (-) to other tick saliva proteomes. The description is the nomenclature of the putative protein, the classification represents the proteins functional category, occurrence represents the number of times the peptides matching to putative proteins were identified using LC-MS/MS when compared to other tick saliva proteomes, and status is a binary representation of when the peptides matching to putative proteins were detected (1) or not (0) when comparing to other tick saliva proteomes.
(XLSX)
A. americanum tick saliva proteins identified using LC-MS/MS from this study were compared to A. americanum unfed saliva proteome. Contig numbers are noted for A. mericanum proteins from this study and from Tirloni et al., (2017) that were present in both proteomes. The description is the nomenclature of the putative protein, the classification represents the proteins functional category, occurrence represents the number of times the peptides matching to putative proteins were identified using LC-MS/MS in unfed non stimulated, host stimulated (dog, human or rabbit), or fed stages every 24 h, and status is a binary representation of when the peptides matching to putative proteins were detected (1) or not (0) in unfed non stimulated, host stimulated (dog, human or rabbit), or fed stages every 24 h. BD represents manually detached ticks that were apparently fully engorged but not yet detached, and SD represents spontaneously detached fully engorged ticks.
(XLSX)
Acknowledgments
The authors would like to thank Dr. José M. C. Ribeiro for providing the VB programs that were used in protein annotations.
Data Availability
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD014844.
Funding Statement
This research was supported by National Institutes of Health grants (AI081093, AI093858, AI074789, AI074789-01A1S1) to AM and National Center for Research Resources (5P41RR011823) and National Institute of General Medical Sciences (8P41GM103533) to JRY. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Dantas-Torres F., Chomel B.B., Otranto D., 2012. Ticks and tick-borne diseases: a One Health perspective. Trends Parasitol. 28, 437–446. 10.1016/j.pt.2012.07.003 [DOI] [PubMed] [Google Scholar]
- 2.Raghavan R.K., Peterson A.T., Cobos M.E., Ganta R., Foley D., 2019. Current and Future Distribution of the Lone Star Tick, Amblyomma americanum (L.) (Acari: Ixodidae) in North America. PLoS One 14, e0209082 10.1371/journal.pone.0209082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson B.E., Sims K.G., Olson J.G., Childs J.E., Piesman J.F., Happ C.M., et al. , 1993. Amblyomma americanum: a potential vector of human ehrlichiosis. Am. J. Trop. Med. Hyg. 49, 239–244. 10.4269/ajtmh.1993.49.239 [DOI] [PubMed] [Google Scholar]
- 4.Buller R.S., Arens M., Hmiel S.P., Paddock C.D., Sumner J.W., Rikhisa Y., et al. , 1999. Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. N. Engl. J. Med. 341, 148–155. 10.1056/NEJM199907153410303 [DOI] [PubMed] [Google Scholar]
- 5.Murphy G.L., Ewing S.A., Whitworth L.C., Fox J.C., Kocan A.A., 1998. A molecular and serologic survey of Ehrlichia canis, E. chaffeensis, and E. ewingii in dogs and ticks from Oklahoma. Vet. Parasitol. 79, 325–339. 10.1016/s0304-4017(98)00179-4 [DOI] [PubMed] [Google Scholar]
- 6.Wolf L., McPherson T., Harrison B., Engber B., Anderson A., Whitt P., 2000. Prevalence of Ehrlichia ewingii in Amblyomma americanum in North Carolina. J. Clin. Microbiol. 38, 2795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hopla CE D.C., 1953. The isolation of Bacterium tularense from the tick Amblyomma americanum. Journal of Kansas Entomological Society 26, 71–72. [Google Scholar]
- 8.Taylor J.P., Istre G.R., McChesney T.C., Satalowich F.T., Parker R.L., McFarland L.M., 1991. Epidemiologic characteristics of human tularemia in the southwest-central states, 1981–1987. Am. J. Epidemiol. 133, 1032–1038. 10.1093/oxfordjournals.aje.a115812 [DOI] [PubMed] [Google Scholar]
- 9.Armstrong P.M., Brunet L.R., Spielman A., Telford S.R.,3rd, 2001. Risk of Lyme disease: perceptions of residents of a Lone Star tick-infested community. Bull. World Health Organ. 79, 916–925. [PMC free article] [PubMed] [Google Scholar]
- 10.James A.M., Liveris D., Wormser G.P., Schwartz I., Montecalvo M.A., Johnson B.J., 2001. Borrelia lonestari infection after a bite by an Amblyomma americanum tick. J. Infect. Dis. 183, 1810–1814. 10.1086/320721 [DOI] [PubMed] [Google Scholar]
- 11.Reeves W.K., Loftis A.D., Nicholson W.L., Czarkowski A.G., 2008. The first report of human illness associated with the Panola Mountain Ehrlichia species: a case report. J. Med. Case Rep. 2, 139 10.1186/1752-1947-2-139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Apperson C.S., Engber B., Nicholson W.L., Mead D.G., Engel J., Yabsley M.J., et al. , 2008. Tick-borne diseases in North Carolina: is "Rickettsia amblyommii" a possible cause of rickettsiosis reported as Rocky Mountain spotted fever? Vector Borne Zoonotic Dis. 8, 597–606. 10.1089/vbz.2007.0271 [DOI] [PubMed] [Google Scholar]
- 13.Breitschwerdt E.B., Hegarty B.C., Maggi R.G., Lantos P.M., Aslett D.M., Bradley J.M., 2011. Rickettsia rickettsii transmission by a lone star tick, North Carolina. Emerg. Infect. Dis. 17, 873–875. 10.3201/eid1705.101530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McMullan L.K., Folk S.M., Kelly A.J., MacNeil A., Goldsmith C.S., Metcalfe M.G., et al. , 2012. A new phlebovirus associated with severe febrile illness in Missouri. N. Engl. J. Med. 367, 834–841. 10.1056/NEJMoa1203378 [DOI] [PubMed] [Google Scholar]
- 15.Savage H.M., Godsey M.S. Jr, Lambert A., Panella N.A., Burkhalter K.L., Harmon J.R., et al. , 2013. First detection of heartland virus (Bunyaviridae: Phlebovirus) from field collected arthropods. Am. J. Trop. Med. Hyg. 89, 445–452. 10.4269/ajtmh.13-0209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinke J.W., Platts-Mills T.A., Commins S.P., 2015. The alpha-gal story: lessons learned from connecting the dots. J. Allergy Clin. Immunol. 135, 96; quiz 597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laird J.S., Kocan A.A., Kocan K.M., Presley S.M., Hair J.A., 1988. Susceptibility of Amblyomma americanum to natural and experimental infections with Theileria cervi. J. Wildl. Dis. 24, 679–683. 10.7589/0090-3558-24.4.679 [DOI] [PubMed] [Google Scholar]
- 18.Little S.E., O'Connor T.P., Hempstead J., Saucier J., Reichard M.V., Meinkoth K., et al. , 2010. Ehrlichia ewingii infection and exposure rates in dogs from the southcentral United States. Vet. Parasitol. 172, 355–360. 10.1016/j.vetpar.2010.05.006 [DOI] [PubMed] [Google Scholar]
- 19.Yabsley M.J., Quick T.C., Little S.E., 2005. Theileriosis in a white-tailed deer (Odocoileus virginianus) fawn. J. Wildl. Dis. 41, 806–809. 10.7589/0090-3558-41.4.806 [DOI] [PubMed] [Google Scholar]
- 20.Barnard D.R., Ervin R.T., Epplin F.M., 1992. Bio-Economic Impact of Amblyomma americanum in Beef Cattle Production Systems. Tick Vector Biology, 55–69. [Google Scholar]
- 21.Tolleson D.R., Teel P.D., Stuth J.W., Strey O.F., Welsh T.H. Jr, Carstens G.E., et al. , 2010. Effects of a lone star tick (Amblyomma americanum) burden on performance and metabolic indicators in growing beef steers. Vet. Parasitol. 173, 99–106. 10.1016/j.vetpar.2010.06.003 [DOI] [PubMed] [Google Scholar]
- 22.Felz M.W., Durden L.A., Oliver J.H.J., 1996. Ticks parasitizing humans in Georgia and South Carolina. The Journal of parasitology 82,3, 505–508. [PubMed] [Google Scholar]
- 23.Nuttall P.A., Labuda M., 2004. Tick-host interactions: saliva-activated transmission. Parasitology 129 Suppl, 177. [DOI] [PubMed] [Google Scholar]
- 24.Bell J.F., Stewart S.J., Wikel S.K., 1979. Resistance to tick-borne Francisella tularensis by tick-sensitized rabbits: allergic klendusity. Am. J. Trop. Med. Hyg. 28, 876–880. [PubMed] [Google Scholar]
- 25.Burke G., Wikel S.K., Spielman A., Telford S.R., McKay K., Krause P.J., Tick-borne Infection Study Group, 2005. Hypersensitivity to ticks and Lyme disease risk. Emerg. Infect. Dis. 11, 36–41. 10.3201/eid1101.040303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nazario S., Das S., de Silva A.M., Deponte K., Marcantonio N., Anderson J.F., et al. , 1998. Prevention of Borrelia burgdorferi transmission in guinea pigs by tick immunity. Am. J. Trop. Med. Hyg. 58, 780–785. 10.4269/ajtmh.1998.58.780 [DOI] [PubMed] [Google Scholar]
- 27.Wikel S.K., Ramachandra R.N., Bergman D.K., Burkot T.R., Piesman J., 1997. Infestation with pathogen-free nymphs of the tick Ixodes scapularis induces host resistance to transmission of Borrelia burgdorferi by ticks. Infect. Immun. 65, 335–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Francis J., Little D.A., 1964. Resistance of Droughtmaster cattle to tick infestation and babesiosis. Aust. Vet. J. 40, 247–253. [Google Scholar]
- 29.Jones L.D., Nuttall P.A., 1990. The effect of host resistance to tick infestation on the transmission of Thogoto virus by ticks. J. Gen. Virol. 71 (Pt 5), 1039–1043. [DOI] [PubMed] [Google Scholar]
- 30.Votiakov V., P Mishaeva N., 1980. Possibility of Protecting Vertebrates Against Transmissive Infection with Tick-Borne Encephalitis Virus. Vopr Virusol. 2,170–173. [PubMed] [Google Scholar]
- 31.Fivaz B.H., Norval R.A., Lawrence J.A., 1989. Transmission of Theileria parva bovis (Boleni strain) to cattle resistant to the brown ear tick Rhipicephalus appendiculatus (Neumann). Trop. Anim. Health Prod. 21, 129–134. 10.1007/bf02236193 [DOI] [PubMed] [Google Scholar]
- 32.Kim T.K., Tirloni L., Pinto A.F., Moresco J., Yates J.R. 3rd, da Silva Vaz I. Jr, et al. , 2016. Ixodes scapularis Tick Saliva Proteins Sequentially Secreted Every 24 h during Blood Feeding. PLoS Negl Trop. Dis. 10, e0004323 10.1371/journal.pntd.0004323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tirloni L., Reck J., Terra R.M., Martins J.R., Mulenga A., Sherman N.E., et al. , 2014. Proteomic Analysis of Cattle Tick Rhipicephalus (Boophilus) microplus Saliva: A Comparison between Partially and Fully Engorged Females. PLoS One 9, e94831 10.1371/journal.pone.0094831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tirloni L., Islam M.S., Kim T.K., Diedrich J.K., Yates J.R.,3rd, Pinto A.F., et al. , 2015. Saliva from nymph and adult females of Haemaphysalis longicornis: a proteomic study. Parasit. Vectors 8, y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oliveira C.J., Anatriello E., de Miranda-Santos I.K., Francischetti I.M., Sa-Nunes A., Ferreira B.R., et al. , 2013. Proteome of Rhipicephalus sanguineus tick saliva induced by the secretagogues pilocarpine and dopamine. Ticks Tick Borne Dis. 4, 469–477. 10.1016/j.ttbdis.2013.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mudenda L., Pierle S.A., Turse J.E., Scoles G.A., Purvine S.O., Nicora C.D., et al. , Brayton K.A., 2014. Proteomics informed by transcriptomics identifies novel secreted proteins in Dermacentor andersoni saliva. Int. J. Parasitol. 44, 1029–1037. 10.1016/j.ijpara.2014.07.003 [DOI] [PubMed] [Google Scholar]
- 37.Diaz-Martin V., Manzano-Roman R., Oleaga A., Encinas-Grandes A., Perez-Sanchez R., 2013. Cloning and characterization of a plasminogen-binding enolase from the saliva of the argasid tick Ornithodoros moubata. Vet. Parasitol. 191, 301–314. 10.1016/j.vetpar.2012.09.019 [DOI] [PubMed] [Google Scholar]
- 38.Tirloni L., Kim T.K., Pinto A.F.M., Yates J.R.,3rd, da Silva Vaz I. Jr, Mulenga A., 2017. Tick-Host Range Adaptation: Changes in Protein Profiles in Unfed Adult Ixodes scapularis and Amblyomma americanum Saliva Stimulated to Feed on Different Hosts. Front. Cell. Infect. Microbiol. 7, 517 10.3389/fcimb.2017.00517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mulenga A., Kim T.K., Ibelli A.M., 2013. Deorphanization and target validation of cross-tick species conserved novel Amblyomma americanum tick saliva protein. Int. J. Parasitol. 43, 439–451. 10.1016/j.ijpara.2012.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim T.K., Curran J., Mulenga A., 2014. Dual silencing of long and short Amblyomma americanum acidic chitinase forms weakens the tick cement cone stability. J. Exp. Biol. 217, 3493–3503. 10.1242/jeb.107979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perez-Riverol Y, Csordas A, Bai J, Bernal-Llinares M, Hewapathirana S, Kundu DJ, et al. , 2019. The PRIDE database and related tools and resources in 2019: improving support for quantification data. Nucleic Acids Res 47(D1):D442–D450. 10.1093/nar/gky1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shevchenko A., Wilm M., Vorm O., Mann M., 1996. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 68, 850–858. 10.1021/ac950914h [DOI] [PubMed] [Google Scholar]
- 43.Porter L., Radulovic Z., Kim T., Braz G.R., Da Silva Vaz I. Jr, Mulenga A., 2015. Bioinformatic analyses of male and female Amblyomma americanum tick expressed serine protease inhibitors (serpins). Ticks Tick Borne Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karim S., Singh P., Ribeiro J.M., 2011. A deep insight into the sialotranscriptome of the gulf coast tick, Amblyomma maculatum. PLoS One 6, e28525 10.1371/journal.pone.0028525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rawlings N.D., Barrett A.J., Bateman A., 2012. MEROPS: the database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 40, 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ashburner M., Ball C.A., Blake J.A., Botstein D., Butler H., Cherry J.M., et al. , 2000. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 25, 25–29. 10.1038/75556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marchler-Bauer A., Derbyshire M.K., Gonzales N.R., Lu S., Chitsaz F., Geer L.Y., et al. , 2015. CDD: NCBI's conserved domain database. Nucleic Acids Res. 43, 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tatusov R.L., Fedorova N.D., Jackson J.D., Jacobs A.R., Kiryutin B., Koonin E.V., et al. , 2003. The COG database: an updated version includes eukaryotes. BMC Bioinformatics 4, 41 10.1186/1471-2105-4-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bateman A., Birney E., Cerruti L., Durbin R., Etwiller L., Eddy S.R., et al. , 2002. The Pfam protein families database. Nucleic Acids Res. 30, 276–280. 10.1093/nar/30.1.276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schultz J., Copley R.R., Doerks T., Ponting C.P., Bork P., 2000. SMART: a web-based tool for the study of genetically mobile domains. Nucleic Acids Res. 28, 231–234. 10.1093/nar/28.1.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fu L., Niu B., Zhu Z., Wu S., Li W., 2012. CD-HIT: accelerated for clustering the next-generation sequencing data. Bioinformatics 28, 3150–3152. 10.1093/bioinformatics/bts565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vizcaino JA, Deutsch EW, Wang R, Csordas A, Reisinger F, et al. (2014) ProteomeXchange provides globally coordinated proteomics data submission and dissemination. Nat Biotechnol 32(3): 223–226. 10.1038/nbt.2839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McDonald W.H., Tabb D.L., Sadygov R.G., MacCoss M.J., Venable J., Graumann J., et al. , 2004. MS1, MS2, and SQT-three unified, compact, and easily parsed file formats for the storage of shotgun proteomic spectra and identifications. Rapid Commun. Mass Spectrom. 18, 2162–2168. 10.1002/rcm.1603 [DOI] [PubMed] [Google Scholar]
- 54.Xu T., Park S.K., Venable J.D., Wohlschlegel J.A., Diedrich J.K., Cociorva D., et al. , 2015. ProLuCID: An improved SEQUEST-like algorithm with enhanced sensitivity and specificity. J. Proteomics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Florens L., Carozza M.J., Swanson S.K., Fournier M., Coleman M.K., Workman J.L., et al. , 2006. Analyzing chromatin remodeling complexes using shotgun proteomics and normalized spectral abundance factors. Methods 40, 303–311. 10.1016/j.ymeth.2006.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paoletti A.C., Parmely T.J., Tomomori-Sato C., Sato S., Zhu D., Conaway R.C., et al. , 2006. Quantitative proteomic analysis of distinct mammalian Mediator complexes using normalized spectral abundance factors. Proc. Natl. Acad. Sci. U. S. A. 103, 18928–18933. 10.1073/pnas.0606379103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu W., Smith J.W., Huang C.M., 2010. Mass spectrometry-based label-free quantitative proteomics. J. Biomed. Biotechnol. 2010, 840518 10.1155/2010/840518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Warnes G.R., Bolker B., Bonebakker L., Gentleman R., Huber W., Liaw A., et al. , 2013. Various R programming tools for plotting data. The Comprehensive R Archive Network. [Google Scholar]
- 59.McSwain J.L., Essenberg R.C., Sauer J.R., 1982. Protein changes in the salivary glands of the female lone star tick, Amblyomma americanum, during feeding. J. Parasitol. 68, 100–106. [PubMed] [Google Scholar]
- 60.Tatchell R.J., 1967. A modified method for obtaining tick oral secretion. J. Parasitol. 53, 1106–1107. [PubMed] [Google Scholar]
- 61.Karim S., Ribeiro J.M., 2015. An Insight into the Sialome of the Lone Star Tick, Amblyomma americanum, with a Glimpse on Its Time Dependent Gene Expression. PLoS One 10, e0131292 10.1371/journal.pone.0131292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ribeiro J.M., Alarcon-Chaidez F., Francischetti I.M., Mans B.J., Mather T.N., Valenzuela J.G., et al. , 2006. An annotated catalog of salivary gland transcripts from Ixodes scapularis ticks. Insect Biochem. Mol. Biol. 36, 111–129. 10.1016/j.ibmb.2005.11.005 [DOI] [PubMed] [Google Scholar]
- 63.Valenzuela J.G., Francischetti I.M., Pham V.M., Garfield M.K., Mather T.N., Ribeiro J.M., 2002. Exploring the sialome of the tick Ixodes scapularis. J. Exp. Biol. 205, 2843–2864. [DOI] [PubMed] [Google Scholar]
- 64.Kemp D.H., Stone B.F., Binnington K.C., 1982. Tick Attachment and Feeding: Role of the Mouthparts, Feeding Apparatus, Salivary Gland Secretions and the Host Response, in Obenchain F., Galun R. (Eds.), Physiology of Ticks. Pergamon Press Ltd, Oxford, UK, pp. 138–139. [Google Scholar]
- 65.des Vignes F., Piesman J., Heffernan R., Schulze T.L., Stafford K.C.,3rd, Fish D., 2001. Effect of tick removal on transmission of Borrelia burgdorferi and Ehrlichia phagocytophila by Ixodes scapularis nymphs. J. Infect. Dis. 183, 773–778. 10.1086/318818 [DOI] [PubMed] [Google Scholar]
- 66.Ebel G.D., Kramer L.D., 2004. Short report: duration of tick attachment required for transmission of powassan virus by deer ticks. Am. J. Trop. Med. Hyg. 71, 268–271. [PubMed] [Google Scholar]
- 67.Porter L.M., Radulovic Z.M., Mulenga A., 2017. A repertoire of protease inhibitor families in Amblyomma americanum and other tick species: inter-species comparative analyses. Parasit. Vectors 10, 1 10.1186/s13071-016-1943-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Blisnick A.A., Foulon T., Bonnet S.I., 2017. Serine Protease Inhibitors in Ticks: An Overview of Their Role in Tick Biology and Tick-Borne Pathogen Transmission. Front. Cell. Infect. Microbiol. 7, 199 10.3389/fcimb.2017.00199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chmelar J., Kotal J., Langhansova H., Kotsyfakis M., 2017. Protease Inhibitors in Tick Saliva: The Role of Serpins and Cystatins in Tick-host-Pathogen Interaction. Front. Cell. Infect. Microbiol. 7, 216 10.3389/fcimb.2017.00216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Law R.H., Zhang Q., McGowan S., Buckle A.M., Silverman G.A., Wong W., et al. , 2006. An overview of the serpin superfamily. Genome Biol. 7, 216 Epub 2006 May 30. 10.1186/gb-2006-7-5-216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mulenga A., Khumthong R., Blandon M.A., 2007a. Molecular and expression analysis of a family of the Amblyomma americanum tick Lospins. J. Exp. Biol. 210, 3188–3198. [DOI] [PubMed] [Google Scholar]
- 72.Mulenga A., Khumthong R., Chalaire K.C., 2009. Ixodes scapularis tick serine proteinase inhibitor (serpin) gene family; annotation and transcriptional analysis. BMC Genomics 10, 217 10.1186/1471-2164-10-217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mulenga A., Sugino M., Nakajim M., Sugimoto C., Onuma M., 2001. Tick-Encoded serine proteinase inhibitors (serpins); potential target antigens for tick vaccine development. J. Vet. Med. Sci. 63, 1063–1069. 10.1292/jvms.63.1063 [DOI] [PubMed] [Google Scholar]
- 74.Mulenga A., Kim T., Ibelli A.M., 2013. Amblyomma americanum tick saliva serine protease inhibitor 6 is a cross-class inhibitor of serine proteases and papain-like cysteine proteases that delays plasma clotting and inhibits platelet aggregation. Insect Mol. Biol. 22, 306–319. 10.1111/imb.12024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim T.K., Tirloni L., Radulovic Z., Lewis L., Bakshi M., Hill C., et al. , 2015. Conserved Amblyomma americanum tick Serpin19, an inhibitor of blood clotting factors Xa and XIa, trypsin and plasmin, has anti-haemostatic functions. Int. J. Parasitol. 45, 613–627. 10.1016/j.ijpara.2015.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tirloni L., Kim T.K., Berger M., Termignoni C., da Silva Vaz I. Jr, Mulenga A., 2019. Amblyomma americanum serpin 27 (AAS27) is a tick salivary anti-inflammatory protein secreted into the host during feeding. PLoS Negl Trop. Dis. 13, e0007660 10.1371/journal.pntd.0007660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Karim S., Miller N.J., Valenzuela J., Sauer J.R., Mather T.N., 2005. RNAi-mediated gene silencing to assess the role of synaptobrevin and cystatin in tick blood feeding. Biochem. Biophys. Res. Commun. 334, 1336–1342. 10.1016/j.bbrc.2005.07.036 [DOI] [PubMed] [Google Scholar]
- 78.Wang X., Shaw D.K., Sakhon O.S., Snyder G.A., Sundberg E.J., Santambrogio L., et al. , 2016. The Tick Protein Sialostatin L2 Binds to Annexin A2 and Inhibits NLRC4-Mediated Inflammasome Activation. Infect. Immun. 84, 1796–1805. 10.1128/IAI.01526-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zavasnik-Bergant T., Vidmar R., Sekirnik A., Fonovic M., Salat J., Grunclova L., et al. , 2017. Salivary Tick Cystatin OmC2 Targets Lysosomal Cathepsins S and C in Human Dendritic Cells. Front. Cell. Infect. Microbiol. 7, 288 10.3389/fcimb.2017.00288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sa-Nunes A., Bafica A., Antonelli L.R., Choi E.Y., Francischetti I.M., Andersen J.F., et al. , 2009. The immunomodulatory action of sialostatin L on dendritic cells reveals its potential to interfere with autoimmunity. J. Immunol. 182, 7422–7429. 10.4049/jimmunol.0900075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang Y., Zhou Y., Gong H., Cao J., Zhang H., Li X., et al. , 2015. Functional characterization of a cystatin from the tick Rhipicephalus haemaphysaloides. Parasit. Vectors 8, 5 10.1186/s13071-014-0620-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wei N., Lin Z., Xu Z., Gong H., Zhang H., Zhou Y., et al. , 2019. Immunosuppressive effects of tick protein RHcyst-1 on murine bone marrow-derived dendritic cells. Parasit. Vectors 12, 1 10.1186/s13071-018-3256-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kopacek P., Weise C., Saravanan T., Vitova K., Grubhoffer L., 2000. Characterization of an alpha-macroglobulin-like glycoprotein isolated from the plasma of the soft tick Ornithodoros moubata. Eur. J. Biochem. 267, 465–475. 10.1046/j.1432-1327.2000.01020.x [DOI] [PubMed] [Google Scholar]
- 84.Buresova V., Hajdusek O., Franta Z., Sojka D., Kopacek P., 2009. IrAM-An alpha2-macroglobulin from the hard tick Ixodes ricinus: characterization and function in phagocytosis of a potential pathogen Chryseobacterium indologenes. Dev. Comp. Immunol. 33, 489–498. 10.1016/j.dci.2008.09.011 [DOI] [PubMed] [Google Scholar]
- 85.Mulenga A., Erikson K., 2011. A snapshot of the Ixodes scapularis degradome. Gene 482, 78–93. 10.1016/j.gene.2011.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fox J.W., Serrano S.M., 2005. Structural considerations of the snake venom metalloproteinases, key members of the M12 reprolysin family of metalloproteinases. Toxicon 45, 969–985. 10.1016/j.toxicon.2005.02.012 [DOI] [PubMed] [Google Scholar]
- 87.Kini R.M., 2006. Anticoagulant proteins from snake venoms: structure, function and mechanism. Biochem. J. 397, 377–387. 10.1042/BJ20060302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Decrem Y., Mariller M., Lahaye K., Blasioli V., Beaufays J., Zouaoui Boudjeltia K., et al. , 2008. The impact of gene knock-down and vaccination against salivary metalloproteases on blood feeding and egg laying by Ixodes ricinus. Int. J. Parasitol. 38, 549–560. 10.1016/j.ijpara.2007.09.003 [DOI] [PubMed] [Google Scholar]
- 89.Francischetti I.M., Mather T.N., Ribeiro J.M., 2003. Cloning of a salivary gland metalloprotease and characterization of gelatinase and fibrin(ogen)lytic activities in the saliva of the Lyme disease tick vector Ixodes scapularis. Biochem. Biophys. Res. Commun. 305, 869–875. 10.1016/s0006-291x(03)00857-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Turner A.J.,2004. Handbook of Proteolytic Enzymes1. [Google Scholar]
- 91.Ewen D., Clarke S.L., Smith J.R., Berger C., Salmon G., Trevethick M., et al. , 2010. The role of protease-activated receptors PAR-1 and PAR-2 in the repair of 16HBE 14o(-) epithelial cell monolayers in vitro. Clin. Exp. Allergy 40, 435–449. 10.1111/j.1365-2222.2010.03453.x [DOI] [PubMed] [Google Scholar]
- 92.Cattaruzza F., Amadesi S., Carlsson J.F., Murphy J.E., Lyo V., Kirkwood K., et al. , 2014. Serine proteases and protease-activated receptor 2 mediate the proinflammatory and algesic actions of diverse stimulants. Br. J. Pharmacol. 171, 3814–3826. 10.1111/bph.12738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sojka D., Francischetti I.M., Calvo E., Kotsyfakis M., 2011. Cysteine proteases from bloodfeeding arthropod ectoparasites. Adv. Exp. Med. Biol. 712, 177–191. 10.1007/978-1-4419-8414-2_11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sun Y., He L., Yu L., Guo J., Nie Z., Liu Q., et al. , 2019. Cathepsin L-a novel cysteine protease from Haemaphysalis flava Neumann, 1897. Parasitol. Res. [DOI] [PubMed] [Google Scholar]
- 95.Zhang T.T., Qiu Z.X., Li Y., Wang W.Y., Li M.M., Guo P., et al. , 2019. The mRNA expression and enzymatic activity of three enzymes during embryonic development of the hard tick Haemaphysalis longicornis. Parasit. Vectors 12, 8 10.1186/s13071-018-3242-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tsuji N., Miyoshi T., Battsetseg B., Matsuo T., Xuan X., Fujisaki K., 2008. A cysteine protease is critical for Babesia spp. transmission in Haemaphysalis ticks. PLoS Pathog. 4, e1000062 10.1371/journal.ppat.1000062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xavier M.A., Tirloni L., Torquato R., Tanaka A., Pinto A.F.M., Diedrich J.K., et al. , 2019. Blood anticlotting activity of a Rhipicephalus microplus cathepsin L-like enzyme. Biochimie 163, 12–20. 10.1016/j.biochi.2019.04.025 [DOI] [PubMed] [Google Scholar]
- 98.Cwiklinski K., de la Torre-Escudero E., Trelis M., Bernal D., Dufresne P.J., Brennan G.P., et al. , 2015. The Extracellular Vesicles of the Helminth Pathogen, Fasciola hepatica: Biogenesis Pathways and Cargo Molecules Involved in Parasite Pathogenesis. Mol. Cell. Proteomics 14, 3258–3273. 10.1074/mcp.M115.053934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Berasain P., Carmona C., Frangione B., Dalton J.P., Goñi F., 2000. Fasciola hepatica: Parasite-Secreted Proteinases Degrade All Human IgG Subclasses: Determination of the Specific Cleavage Sites and Identification of the Immunoglobulin Fragments Produced. Exp Parasitol. 94, 99–110. 10.1006/expr.1999.4479 [DOI] [PubMed] [Google Scholar]
- 100.Robinson M.W., Corvo I., Jones P.M., George A.M., Padula M.P., To J., et al. , 2011. Collagenolytic activities of the major secreted cathepsin L peptidases involved in the virulence of the helminth pathogen, Fasciola hepatica. PLoS Negl Trop. Dis. 5, e1012 10.1371/journal.pntd.0001012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Akov S., 1982. Blood Digestion in Ticks. pp 197–212. Obenchain F.D and Galun R., Physiology of Ticks. [Google Scholar]
- 102.Boldbaatar D., Sikalizyo Sikasunge C., Battsetseg B., Xuan X., Fujisaki K., 2006. Molecular cloning and functional characterization of an aspartic protease from the hard tick Haemaphysalis longicornis. Insect Biochem. Mol. Biol. 36, 25–36. 10.1016/j.ibmb.2005.10.003 [DOI] [PubMed] [Google Scholar]
- 103.Logullo C., Vaz Ida S., Sorgine M.H., Paiva-Silva G.O., Faria F.S., Zingali R.B., et al. , 1998. Isolation of an aspartic proteinase precursor from the egg of a hard tick, Boophilus microplus. Parasitology 116 (Pt 6), 525–532. [DOI] [PubMed] [Google Scholar]
- 104.Sorgine M.H., Logullo C., Zingali R.B., Paiva-Silva G.O., Juliano L., Oliveira P.L., 2000. A heme-binding aspartic proteinase from the eggs of the hard tick Boophilus microplus. J. Biol. Chem. 275, 28659–28665. 10.1074/jbc.M005675200 [DOI] [PubMed] [Google Scholar]
- 105.Paesen G.C., Adams P.L., Harlos K., Nuttall P.A., Stuart D.I., 1999. Tick histamine-binding proteins: isolation, cloning, and three-dimensional structure. Mol. Cell 3, 661–671. 10.1016/s1097-2765(00)80359-7 [DOI] [PubMed] [Google Scholar]
- 106.Fluckinger M., Haas H., Merschak P., Glasgow B.J., Redl B., 2004. Human tear lipocalin exhibits antimicrobial activity by scavenging microbial siderophores. Antimicrob. Agents Chemother. 48, 3367–3372. 10.1128/AAC.48.9.3367-3372.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chan Y.R., Liu J.S., Pociask D.A., Zheng M., Mietzner T.A., Berger T., et al. , 2009. Lipocalin 2 is required for pulmonary host defense against Klebsiella infection. J. Immunol. 182, 4947–4956. 10.4049/jimmunol.0803282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cho K.W., Zhou Y., Sheng L., Rui L., 2011. Lipocalin-13 regulates glucose metabolism by both insulin-dependent and insulin-independent mechanisms. Mol. Cell. Biol. 31, 450–457. 10.1128/MCB.00459-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ganfornina M.D., Kayser H., Sanchez D., 2000. Lipocalins in Arthropoda: Diversification and Functional Explorations In: Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience. [Google Scholar]
- 110.Smathers R.L., Petersen D.R., 2011. The human fatty acid-binding protein family: evolutionary divergences and functions. Hum. Genomics 5, 170–191. 10.1186/1479-7364-5-3-170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Koreny L., Obornik M., Lukes J., 2013. Make it, take it, or leave it: heme metabolism of parasites. PLoS Pathog. 9, e1003088 10.1371/journal.ppat.1003088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Braz G.R., Coelho H.S., Masuda H., Oliveira P.L., 1999. A missing metabolic pathway in the cattle tick Boophilus microplus. Curr. Biol. 9, 703–706. 10.1016/s0960-9822(99)80312-1 [DOI] [PubMed] [Google Scholar]
- 113.Perner J., Sobotka R., Sima R., Konvickova J., Sojka D., Oliveira P.L., et al. , 2016. Acquisition of exogenous haem is essential for tick reproduction. Elife 5, 10.7554/eLife.12318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Toh S.Q., Glanfield A., Gobert G.N., Jones M.K., 2010. Heme and blood-feeding parasites: friends or foes? Parasit. Vectors 3, 108 10.1186/1756-3305-3-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lara F.A., Lins U., Bechara G.H., Oliveira P.L., 2005. Tracing heme in a living cell: hemoglobin degradation and heme traffic in digest cells of the cattle tick Boophilus microplus. J. Exp. Biol. 208, 3093–3101. 10.1242/jeb.01749 [DOI] [PubMed] [Google Scholar]
- 116.Maya-Monteiro C.M., Daffre S., Logullo C., Lara F.A., Alves E.W., Capurro M.L., et al. , 2000. HeLp, a heme lipoprotein from the hemolymph of the cattle tick, Boophilus microplus. J. Biol. Chem. 275, 36584–36589. 10.1074/jbc.M007344200 [DOI] [PubMed] [Google Scholar]
- 117.Gudderra N.P., Sonenshine D.E., Apperson C.S., Roe R.M., 2002a. Hemolymph proteins in ticks. Journal of Insect Physiology 48, 269–278. [DOI] [PubMed] [Google Scholar]
- 118.Donohue K.V., Khalil S.M., Mitchell R.D., Sonenshine D.E., Roe R.M., 2008. Molecular characterization of the major hemelipoglycoprotein in ixodid ticks. Insect Mol Biol 17: 197–208. 10.1111/j.1365-2583.2008.00794.x [DOI] [PubMed] [Google Scholar]
- 119.Dutra F.F., Bozza M.T., 2014. Heme on innate immunity and inflammation. Front. Pharmacol. 5, 115 10.3389/fphar.2014.00115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Maya-Monteiro C.M., Alves L.R., Pinhal N., Abdalla D.S., Oliveira P.L., 2004. HeLp, a heme-transporting lipoprotein with an antioxidant role. Insect Biochem. Mol. Biol. 34, 81–88. 10.1016/j.ibmb.2003.09.005 [DOI] [PubMed] [Google Scholar]
- 121.Seixas A., Alzugaray M.F., Tirloni L., Parizi L.F., Pinto A.F.M., Githaka N.W., et al. , 2018. Expression profile of Rhipicephalus microplus vitellogenin receptor during oogenesis. Ticks Tick Borne Dis. 9, 72–81. 10.1016/j.ttbdis.2017.10.006 [DOI] [PubMed] [Google Scholar]
- 122.Hussein H.E., Johnson W.C., Taus N.S., Suarez C.E., Scoles G.A., Ueti M.W., 2019. Silencing expression of the Rhipicephalus microplus vitellogenin receptor gene blocks Babesia bovis transmission and interferes with oocyte maturation. Parasit. Vectors 12, 1 10.1186/s13071-018-3256-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Narasimhan S., Deponte K., Marcantonio N., Liang X., Royce T.E., Nelson K.F., et al. , 2007. Immunity against Ixodes scapularis salivary proteins expressed within 24 hours of attachment thwarts tick feeding and impairs Borrelia transmission. PLoS One 2, e451 10.1371/journal.pone.0000451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rojkind M., Dominguez-Rosales J.A., Nieto N., Greenwel P., 2002. Role of hydrogen peroxide and oxidative stress in healing responses. Cell Mol. Life Sci. 59, 1872–1891. 10.1007/pl00012511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Adamson S., Browning R., Singh P., Nobles S., Villarreal A., Karim S., 2014. Transcriptional activation of antioxidants may compensate for selenoprotein deficiencies in Amblyomma maculatum (Acari: Ixodidae) injected with selK- or selM-dsRNA. Insect Mol. Biol. 23, 497–510. 10.1111/imb.12098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kumar D., Budachetri K., Meyers V.C., Karim S., 2016. Assessment of tick antioxidant responses to exogenous oxidative stressors and insight into the role of catalase in the reproductive fitness of the Gulf Coast tick, Amblyomma maculatum. Insect Mol. Biol. 25, 283–294. 10.1111/imb.12218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Das S., Banerjee G., DePonte K., Marcantonio N., Kantor F.S., Fikrig E., 2001. Salp25D, an Ixodes scapularis antioxidant, is 1 of 14 immunodominant antigens in engorged tick salivary glands. J. Infect. Dis. 184, 1056–1064. 10.1086/323351 [DOI] [PubMed] [Google Scholar]
- 128.Wu J., Wang Y., Liu H., Yang H., Ma D., Li J., et al. , 2010. Two immunoregulatory peptides with antioxidant activity from tick salivary glands. J. Biol. Chem. 285, 16606–16613. 10.1074/jbc.M109.094615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lewis L.A., Radulovic Z.M., Kim T.K., Porter L.M., Mulenga A., 2015. Identification of 24h Ixodes scapularis immunogenic tick saliva proteins. Ticks Tick Borne Dis. 6, 424–434. 10.1016/j.ttbdis.2015.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Radulovic Z.M., Kim T.K., Porter L.M., Sze S.H., Lewis L., Mulenga A., 2014. A 24–48 h fed Amblyomma americanum tick saliva immuno-proteome. BMC Genomics 15, 518 10.1186/1471-2164-15-518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Binnington K.C., Kemp D.H., 1980. Role of tick salivary glands in feeding and disease transmission. Adv. Parasitol. 18, 315–339. 10.1016/s0065-308x(08)60403-0 [DOI] [PubMed] [Google Scholar]
- 132.Sonenshine D.E., 1993. Biology of Ticks. Oxford University Press, Oxford. [Google Scholar]
- 133.Alekseev A.N., Burenkova L.A., Podboronov V.M., Chunikhin S.P., 1995. Bacteriocidal qualities of ixodid tick (Acarina: Ixodidae) salivary cement plugs and their changes under the influence of a viral tick-borne pathogen. J. Med. Entomol. 32, 578–582. 10.1093/jmedent/32.5.578 [DOI] [PubMed] [Google Scholar]
- 134.Bishop R., Lambson B., Wells C., Pandit P., Osaso J., Nkonge C., et al. , 2002. A cement protein of the tick Rhipicephalus appendiculatus, located in the secretory e cell granules of the type III salivary gland acini, induces strong antibody responses in cattle. Int. J. Parasitol. 32, 833–842. 10.1016/s0020-7519(02)00027-9 [DOI] [PubMed] [Google Scholar]
- 135.Bullard R., Allen P., Chao C.C., Douglas J., Das P., Morgan S.E., et al. , 2016. Structural characterization of tick cement cones collected from in vivo and artificial membrane blood-fed Lone Star ticks (Amblyomma americanum). Ticks Tick Borne Dis. 7, 880–892. 10.1016/j.ttbdis.2016.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hollmann T., Kim T.K., Tirloni L., Radulovic Z.M., Pinto A.F.M., Diedrich J.K., et al. , 2017. Identification and characterization of proteins in the Amblyomma americanum tick cement cone. Int. J. Parasitol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mulenga A., Sugimoto C., Sako Y., Ohashi K., Musoke A., Shubash M., et al. , 1999. Molecular characterization of a Haemaphysalis longicornis tick salivary gland-associated 29-kilodalton protein and its effect as a vaccine against tick infestation in rabbits. Infect. Immun. 67, 1652–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Czolpinska M., Rurek M., 2018. Plant Glycine-Rich Proteins in Stress Response: An Emerging, Still Prospective Story. Front. Plant. Sci. 9, 302 10.3389/fpls.2018.00302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rossi D.C.P., Silva F.D., Daffre S., Martinez L.R., Nosanchuk J.D., Frases S., et al. , 2011. Effects of microplusin, a copper-chelating antimicrobial peptide, against Cryptococcus neoformans. femsle 324, 64–72. [DOI] [PubMed] [Google Scholar]
- 140.Silva F.D., Rezende C.A., Rossi D.C., Esteves E., Dyszy F.H., Schreier S., et al. , 2009. Structure and mode of action of microplusin, a copper II-chelating antimicrobial peptide from the cattle tick Rhipicephalus (Boophilus) microplus. J. Biol. Chem. 284, 34735–34746. 10.1074/jbc.M109.016410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nakajima Y., van der Goes van Naters-Yasui A, Taylor D., Yamakawa M., 2003. Antibacterial peptide defensin is involved in midgut immunity of the soft tick, Ornithodoros moubata. Insect Mol. Biol. 11, 611–618. [DOI] [PubMed] [Google Scholar]
- 142.Petrou G., Crouzier T., 2018. Mucins as multifunctional building blocks of biomaterials. Biomater. Sci. 6, 2282–2297. 10.1039/c8bm00471d [DOI] [PubMed] [Google Scholar]
- 143.Eaton J.R.O., Alenazi Y., Singh K., Davies G., Geis-Asteggiante L., Kessler B., et al. , 2018. The N-terminal domain of a tick evasin is critical for chemokine binding and neutralization and confers specific binding activity to other evasins. J. Biol. Chem. 293, 6134–6146. 10.1074/jbc.RA117.000487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hayward J., Sanchez J., Perry A., Huang C., Rodriguez Valle M., Canals M., et al. , 2017. Ticks from diverse genera encode chemokine-inhibitory evasin proteins. J. Biol. Chem. 292, 15670–15680. 10.1074/jbc.M117.807255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Francischetti I.M., My Pham V., Mans B.J., Andersen J.F., Mather T.N., Lane R.S., et al. , 2005. The transcriptome of the salivary glands of the female western black-legged tick Ixodes pacificus (Acari: Ixodidae). Insect Biochem. Mol. Biol. 35, 1142–1161. 10.1016/j.ibmb.2005.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Smith S.A., Travers R.J., Morrissey J.H., 2015. How it all starts: Initiation of the clotting cascade. Crit. Rev. Biochem. Mol. Biol. 50, 326–336. 10.3109/10409238.2015.1050550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Konnai S., Nakajima C., Imamura S., Yamada S., Nishikado H., Kodama M., et al. , 2009. Suppression of cell proliferation and cytokine expression by HL-p36, a tick salivary gland-derived protein of Haemaphysalis longicornis. Immunology 126, 209–219. 10.1111/j.1365-2567.2008.02890.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mulenga A., Blandon M., Khumthong R., 2007b. The molecular basis of the Amblyomma americanum tick attachment phase. Exp. Appl. Acarol. 41, 267–287. [DOI] [PubMed] [Google Scholar]
- 149.Francischetti I.M., Sa-Nunes A., Mans B.J., Santos I.M., Ribeiro J.M., 2009. The role of saliva in tick feeding. Front. Biosci. (Landmark Ed) 14, 2051–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rock K.L., Kono H., 2008. The inflammatory response to cell death. Annu. Rev. Pathol. 3, 99–126. 10.1146/annurev.pathmechdis.3.121806.151456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zininga T., Ramatsui L., Shonhai A., 2018. Heat Shock Proteins as Immunomodulants. Molecules 23, 10.3390/molecules23112846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Poirier A.C., Schmitt P., Rosa R.D., Vanhove A.S., Kieffer-Jaquinod S., Rubio T.P., et al. , 2014. Antimicrobial histones and DNA traps in invertebrate immunity: evidences in Crassostrea gigas. J. Biol. Chem. 289, 24821–24831. 10.1074/jbc.M114.576546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Puente X.S., Lopez-Otin C., 2004. A genomic analysis of rat proteases and protease inhibitors. Genome Res. 14, 609–622. 10.1101/gr.1946304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shah D., Mital K., 2018. The Role of Trypsin:Chymotrypsin in Tissue Repair. Adv. Ther. 35, 31–42. 10.1007/s12325-017-0648-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mosesson M.W., 2005. Fibrinogen and fibrin structure and functions. J. Thromb. Haemost. 3, 1894–1904. 10.1111/j.1538-7836.2005.01365.x [DOI] [PubMed] [Google Scholar]
- 156.Abdollahi M., Ng T.S., Rezaeizadeh A., Aamidor S., Twigg S.M., Min D., et al. , 2017. Insulin treatment prevents wounding associated changes in tissue and circulating neutrophil MMP-9 and NGAL in diabetic rats. PLoS One 12, e0170951 10.1371/journal.pone.0170951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Smout M.J., Sotillo J., Laha T., Papatpremsiri A., Rinaldi G., Pimenta R.N., et al. , 2015. Carcinogenic parasite secretes growth factor that accelerates wound healing and potentially promotes neoplasia. PLoS Pathog. 11, e1005209 10.1371/journal.ppat.1005209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Black W.C.,4th, Piesman J., 1994. Phylogeny of hard- and soft-tick taxa (Acari: Ixodida) based on mitochondrial 16S rDNA sequences. Proc. Natl. Acad. Sci. U. S. A. 91, 10034–10038. 10.1073/pnas.91.21.10034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hoogstraal H., Aeschilimann A., 1982. Tick-host specificity. Mitt. Schweiz. Entomol. Ges. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Normalized spectral abundance factors (NSAF) values of tick saliva proteins that did not show similarity to housekeeping proteins were normalized using the z-score statistics and then used to generate heat maps using heatmap2 function in gplots library using R as described in materials and methods. (Protease Inhibitors are labeled as A- Serpins, B- Cystatins, C- ⍺2-macroglobulin, D-Kunitz type, E- trypsin inhibitor like; Protease are labeled as F- cysteine, G- metalloprotease, H- serine; and other protein classes as I- Lipocalin, J- heme/iron binding, K- antioxidants, L- glycine rich, M- extracellular matrix, N- antimicrobial, O- Mucin/ mucin-like, P- Evasin, Q- Ixodegrin, R- Immune related, and S- tick specific secreted saliva proteins of unknown function).
(TIFF)
The peptide count, spectral count, normalized spectral abundance factor (NSAF), exponentially modified protein abundance index (EMPAI), spectral count, and sequence coverage (%) generated from the Prolucid and IDcompare analyses are represented on an excel file with different tablatures for tick, rabbit host, contaminants, and reversed-sequences. The time point during tick feeding is noted for every 24 h, BD represents manually detached ticks that were apparently fully engorged but not yet detached, and SD represents spontaneously detached fully engorged ticks. The contig represents the identifier for the CDS extracted from the assembled BioProject accession # PRJNA226980. The description is the nomenclature of the putative protein, the classification represents the proteins functional category, occurrence represents the number of times the peptides matching to putative proteins were identified using LC-MS/MS during feeding time points, and status is a binary representation of when the peptides matching to putative proteins were detected (1) or not (0) during feeding time points.
(XLSX)
A. americanum tick saliva proteins identified using LC-MS/MS from this study were compared to currently published A. americanum ticks saliva proteomes. The peptide count, spectral count, normalized spectral abundance factor (NSAF), exponentially modified protein abundance index (EMPAI), spectral count, and sequence coverage (%) generated from the Prolucid and IDcompare analyses are represented on an excel file. The time point during tick feeding is noted for every 24 h, BD represents manually detached ticks that were apparently fully engorged but not yet detached, and SD represents spontaneously detached fully engorged ticks. The identifier represents the contig for the CDS extracted from the assembled BioProject accession # PRJNA226980, or from NCBI GenBank accession numbers. Identifiers with no matches were noted with N/A. The description is the nomenclature of the putative protein, the classification represents the proteins functional category, occurrence represents the number of times the peptides matching to putative proteins were identified using LC-MS/MS during feeding time points, and status is a binary representation of when the peptides matching to putative proteins were detected (1) or not (0) during feeding time points.
(XLSX)
A. americanum tick saliva proteins identified using LC-MS/MS from this study were compared to currently published tick saliva proteomes of I. scapularis, H. longicornis, R. microplus, D. andersoni, O. moubata, and R. sanguineus. Contig numbers are noted for A. mericanum proteins that matched (+) or not matched (-) to other tick saliva proteomes. The description is the nomenclature of the putative protein, the classification represents the proteins functional category, occurrence represents the number of times the peptides matching to putative proteins were identified using LC-MS/MS when compared to other tick saliva proteomes, and status is a binary representation of when the peptides matching to putative proteins were detected (1) or not (0) when comparing to other tick saliva proteomes.
(XLSX)
A. americanum tick saliva proteins identified using LC-MS/MS from this study were compared to A. americanum unfed saliva proteome. Contig numbers are noted for A. mericanum proteins from this study and from Tirloni et al., (2017) that were present in both proteomes. The description is the nomenclature of the putative protein, the classification represents the proteins functional category, occurrence represents the number of times the peptides matching to putative proteins were identified using LC-MS/MS in unfed non stimulated, host stimulated (dog, human or rabbit), or fed stages every 24 h, and status is a binary representation of when the peptides matching to putative proteins were detected (1) or not (0) in unfed non stimulated, host stimulated (dog, human or rabbit), or fed stages every 24 h. BD represents manually detached ticks that were apparently fully engorged but not yet detached, and SD represents spontaneously detached fully engorged ticks.
(XLSX)
Data Availability Statement
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD014844.