Abstract
Chronic lung allograft dysfunction (CLAD), a condition of excess matrix deposition and airways fibrosis, limits survival after lung transplantation. Amphiregulin (Areg) is an epidermal growth factor receptor (EGFR) ligand suggested to regulate airway injury and repair. We sought to determine whether Areg expression increases in CLAD, localize the cellular source of Areg induction in CLAD, and assess its effects on airway matrix deposition. Lung fluid Areg protein was quantified in patients with or without CLAD. In situ hybridization was performed to localize Areg and EGFR transcript in CLAD and normal lung tissue. Expression of hyaluronan, a matrix constituent that accumulates in CLAD, was measured in Areg exposed bronchial epithelial cells in the presence or absence of an EGFR inhibitor. We demonstrated lung fluid Areg protein was significantly increased in CLAD in a discovery and replication cohort. Areg and EGFR transcripts were abundantly expressed within CLAD tissue; localized to basally distributed airway epithelial cells overlying fibrotic regions. Areg exposed bronchial epithelial cells increased hyaluronan and hyaluronan synthase expression in an EGFR dependent manner. Collectively, these novel observations suggest Areg contributes to airway remodeling and CLAD. Moreover these data implicate a role for EGFR signaling in CLAD pathogenesis, suggesting novel therapeutic targets.
1. INTRODUCTION
Lung transplantation is an established therapy for end-stage pulmonary disease. Long-term outcomes however remain limited due to chronic lung allograft dysfunction (CLAD)1. CLAD is characterized by an irreversible decline in pulmonary function and histologically correlates with lung fibrosis. The most common histological manifestation of CLAD is airways fibrosis, or obliterative bronchiolitis (OB). Importantly, studies suggest OB is a histopathological feature observed in both described CLAD phenotypes, bronchiolitis obliterans syndrome (BOS) and restrictive CLAD (R-CLAD)2,3. While the mechanisms underlying OB development in lung recipients are not precisely defined, OB is felt to represent the shared outcome of immune and non-immune mediated insults to the airway epithelium. Thus, suggesting a disease development paradigm characterized by epithelial injury, altered epithelial cell function, and airway remodeling with extracellular matrix (ECM) accumulation and intraluminal fibroproliferation4. Consistent with this idea, our prior work demonstrated the ECM constituent, hyaluronan (HA), deposits within fibrotic airways in CLAD and furthermore suggested HA synthetic enzymes are upregulated in CLAD tissue5
Amphiregulin (Areg), an epidermal growth factor receptor (EGFR) ligand, has been implicated in tissue repair and fibrosis6–8. In vivo studies demonstrated intrapulmonary Areg is upregulated in mice with bleomycin induced lung fibrosis and in vitro data suggests human bronchial epithelial cells shed Areg upon exposure to a range of toxins9,10. We recently showed Areg protein and transcript are increased in a rodent model of toxin-induced OB11. Specifically, Areg overexpression was localized to bronchial epithelial cells in airways affected by OB, suggesting a role for Areg in the progression from epithelial cell injury to fibrotic airway remodeling. No prior studies, however, have evaluated Areg in the context of human OB developing after lung transplantation.
As experimental data support a role for Areg in lung fibrosis we sought to evaluate the role of Areg in human lung recipients with a clinical features of CLAD or histological OB. In particular, the objectives of this study were to 1) determine whether Areg is increased in the allograft fluid or tissue in CLAD, 2) localize the cellular source of Areg induction, and 3) evaluate the effects of Areg and EGFR signaling on airway ECM deposition, specifically, synthesis and accumulation of HA.
2. MATERIALS AND METHODS
2.1. Lung fluid cohorts for Areg protein quantification
Independent cohorts were drawn from first, adult single or bilateral lung recipients at Duke University Medical Center (DUMC) or the University of California Los Angeles (UCLA), respectively. Patients were managed according to center-specific protocols (supplement). Bronchoalveolar lavage (BAL) samples were prospectively collected during surveillance or clinically indicated bronchoscopy. Samples were obtained, processed, and stored as previously published12,13. CLAD was defined as a sustained ≥20% decline in forced expiratory volume in 1 second compared with the average of the two best posttransplant values in the absence of clinical confounders14 and was further phenotyped to BOS or R-CLAD at onset according to published methodology15. Samples were excluded if there was concurrent acute rejection on biopsy or infection on culture.
BAL samples from 114 patients were included in the cross-sectional DUMC cohort; CLAD free (n=59) and CLAD (n=55; 44 BOS and 11 R-CLAD). Of the 55 CLAD patients, 14 had a BAL sample banked prior to CLAD permitting comparison of Areg levels prior to and after the onset of CLAD in this subset. BAL samples from 54 patients were included in the cross-sectional UCLA cohort; CLAD free (n=12) and CLAD (n=42; 19 BOS and 23 R-CLAD). Of the 42 patients with CLAD, 21 had a sample banked prior to CLAD and comprised the subset for UCLA longitudinal analysis.
Table S1 specifies catalog information for all reagents. Areg protein was measured in DUMC BAL samples using a commercial multiplex (EMD Millipore, Burlington, MA; reliable limit of Areg detection 4.74pg/mL) that assessed for multiple EGFR ligands. Samples were analyzed undiluted and those with concentrations falling below the range of detection (n=16 for Areg) were assigned a concentration of the lowest detectable value. At UCLA, BAL Areg was quantified using commercial ELISA (R&D Systems, Minneapolis, MN; reliable limit of Areg detection 15.6pg/mL). Samples were analyzed undiluted and those with concentrations falling below the range of reliable detection (n=27) were assigned a concentration of the lower limit of detection (average of the blanks plus two standard deviations). All studies were approved by the Internal Review Board; Pro00022225 and Pro00013378 at Duke and 2515–0085 at UCLA.
2.2. Whole lung transcript cohort
Lung tissue was procured at the time of retransplantation for advanced CLAD at DUMC. Unused donor tissue, trimmed at the time of transplant due to size mismatch and demonstrated to be normal on histopathological examination, was used as normal control lung tissue. Tissue RNA (CLAD explant n=17, 15 BOS and 2 R-CLAD; normal donor n=12) was preserved, isolated, reverse transcribed, and Taqman assays for Areg and EGFR were performed as detailed in the supplement.
2.3. Chromogenic in situ hybridization (CISH)
Formalin fixed paraffin embedded tissue from CLAD explant (n=4, all BOS) and normal donor (n=2) was sectioned 24–48hrs before use and stored at 4°C until time of processing. Probes of interest including Areg, EGFR, PPIB (positive control) and negative control were purchased from Advanced Cell Diagnostics (Newark, CA). Slides were processed according to manufacturer instructions (supplement). Probes were developed using chromogenic red kit. Images were obtained using a Zeiss Observer.Z1 inverted fluorescent microscope (Carl Zeiss, Inc., Gottingen, Germany) equipped with a digital camera and processed using AxioVision Release 4.6.3 (Carl Zeiss) Software.
2.4. Cell culture
NCI-H292 bronchial epithelial cells were split at ~85% confluence for several passages and seeded at 200,000 cells per well of 12 well plate 18 to 24 hours prior to use. Cells were rinsed once with serum free media and treated with Areg (0, 3, 10, or 30 ng/mL) in serum free media for 6 or 24 hours in the absence or presence of the 10 μM AG1478, an EGFR inhibitor. Supernatants were collected, centrifuged at 15,000 rpm for 10 minutes, and stored at −20°C. Hyaluronan (HA) concentrations were measured in the supernatants using commercial ELISA (R&D Systems, Minneapolis, MN). Cells were lysed with Trizol and RNA was extracted using phenol chloroform extraction with RNeasy columns (Quiagen, Germantown, MD). RNA was reverse transcribed into cDNA using the high capacity reverse transcription kit (ThermoFisher, Waltham, MA). Transcipt for the HA synthetic enzyme, HA synthase 2 (HAS2) (ThermoFisher, Waltham, MA), was assessed by RT-PCR using 40ng of cDNA in each assay. Experiments were completed in triplicate and a representative experiment analyzed.
2.5. Statistical analysis
Nonparametric t-tests were used to assess differences in characteristics between patients with or without CLAD; Chi-square or Fisher’s Exact tests, as appropriate, were used to evaluate frequency differences. Data are reported as mean ± standard error. Unpaired t-test (with correction for unequal variances, as appropriate) was applied to assess the difference in mean BAL (cross-sectional cohorts) Areg levels between CLAD-free and CLAD samples and to assess the difference in the mean fold-change in Areg or EGFR transcript between normal donor and CLAD tissues. A Wilcoxon matched-pairs signed rank test was used to assess the differences in median BAL Areg levels between antecedent CLAD samples and post-CLAD samples in the longitudinal BAL cohorts. Cell culture ELISA and transcript results at each time point in the absence of inhibitor was analyzed using one way ANOVA with Bonferroni correction for multiple comparisons to compare untreated to Areg treated cells at each dose. Unpaired t-test (with correction for unequal variances, as appropriate) was applied to compare untreated vs. Ag-1478 treated samples. Statistical analysis was performed using Graph Pad Prism 8.0.2 (GraphPad Software Inc, La Jolla, CA).
3. RESULTS
3.1. Amphiregulin Protein is Increased in CLAD
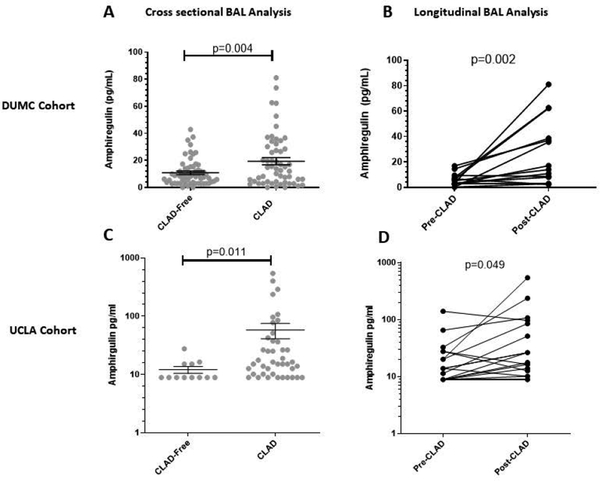
We first sought to determine whether BAL Areg protein is increased in association with CLAD in both a discovery and independent replication cohort (Table 1). Within the DUMC cohort, BAL Areg protein was significantly higher in lung recipients with as compared to those without CLAD at collection (19.4 ± 2.6pg/mL vs. 10.9 ± 1.3pg/mL, p=0.004) (Figure 1a). Furthermore, Areg concentrations increased significantly within the same patient after the onset of CLAD (median increase 11.6pg/mL, p=0.002) (Figure 1b). Similar findings were observed in the UCLA cohort with a mean BAL Areg protein concentration of 58.2 ± 17.1pg/mL in those with CLAD vs. 12.1 ± 1.7pg/mL in those without CLAD, p=0.011 (Figure 1c). Moreover, patients sampled before and after the onset of CLAD demonstrated a median increase in BAL Areg protein of 5.0 pg/mL, p=0.049 (Figure 1d). In both cohorts, BAL Areg concentrations were similar among patients with CLAD, irrespective of CLAD phenotype (Figure S1). Additionally, no statistically significant differences were observed in any of the other EGFR ligands assessed in the BAL, and many were poorly detectable (Table S2).
Table 1.
Cross-sectional bronchoalveolar lavage cohort demographics.
| DUMC Cohort | ||||
|---|---|---|---|---|
| Characteristic | CLAD Free (N=59) n, [%] |
CLAD (N=55) n, [%] |
p-value | |
| Age at Transplant(y)* | 52 [44, 63] | 52 [37, 64] | 0.831 | |
| Caucasian Race | 51 [86] | 47 [85] | >0.999 | |
| Female Gender | 27 [46] | 18 [33] | 0.182 | |
| Transplant | COPD | 22 [37] | 21 [38] | >0.999 |
| 17 [29] | 14 [25] | 0.834 | ||
| 11 [19] | 10 [18] | >0.999 | ||
| 9 [15] | 10 [18] | 0.803 | ||
| Bilateral Lung Transplant | 57 [97] | 48 [87] | 0.086 | |
| Time from Transplant to CLAD Onset, days* | --- | 1391 [940, 2483] | --- | |
| Time from Transplant to Sample, days* | 1335 [1083, 1959] | 2253 [1224, 3079] | 0.002 | |
| Time from CLAD to Sample, days* | --- | 232 [114, 872] | --- | |
| UCLA Cohort | ||||
| Characteristic | CLAD Free (N=12) n, [%] |
CLAD (N=42) n, [%] |
p-value | |
| Age at Transplant(y)* | 60 [36, 64] | 58 [46, 62] | 0.785 | |
| Caucasian Race | 7 [58] | 30 [71] | 0.486 | |
| Female Gender | 8 [67] | 22 [52] | 0.515 | |
| Transplant | COPD | 4 [33] | 11 [26] | 0.719 |
| 5 [42] | 24 [57] | 0.513 | ||
| 1 [8] | 1 [2] | 0.398 | ||
| 2 [17] | 6 [14] | >0.999 | ||
| Bilateral Lung Transplant | 6 [50] | 31 [74] | 0.162 | |
| Time from Transplant to CLAD Onset, days* | --- | 603 [367, 770] | --- | |
| Time from Transplant to Sample, days* | 281 [181, 377] | 704 [444, 1094] | <0.0001 | |
| Time from CLAD to Sample, days* | --- | 48 [14, 141] | --- | |
Median [IQR]
DUMC = Duke University Medical Center, CLAD = chronic lung allograft dysfunction, COPD = chronic obstructive pulmonary disease, UCLA = University of California Los Angeles
Figure 1.
Amphiregulin levels are significantly elevated in bronchoalveolar lavage (BAL) fluid from patients with, as compared to those without, CLAD at the time of collection. A) Cross-sectional BAL data from DUMC represented as CLAD free patients (n=59) compared to patients with CLAD at the time of BAL collection (n=55). B) Longitudinal BAL data from DUMC before and after the onset of CLAD in a subset of patients sampled serially after transplantation (n=14). C) Cross-sectional BAL data from UCLA represented as CLAD free patients (n=12) compared to patients with CLAD at the time of BAL collection (n=42). B) Longitudinal BAL data from UCLA before and after the onset of CLAD in a subset of patients sampled serially after transplantation (n=21). For the cross-sectional data individual data points are represented as circles and the mean and standard error of the mean are represented by the black horizontal or vertical bars, respectively.
BAL=bronchoalveolar lavage; CLAD=chronic lung allograft dysfunction; DUMC-Duke University Medical Center; UCLA=University of California Los Angeles
3.2. Amphiregulin and EGFR Transcript are Increased in CLAD
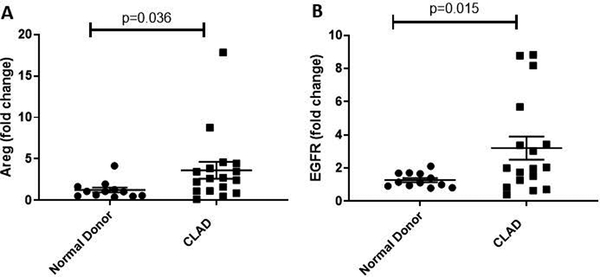
We next sought to further determine whether pulmonary Areg production is upregulated in association with CLAD. Whole lung transcript analysis demonstrated Areg mRNA expression was significantly increased in CLAD affected lung tissue when compared to that of normal donor controls (3.65-fold ± 1.02 vs. 1.26 ± 0.30, p=0.036) (Figure 2a). As Areg signals through EGFR, we also examined whole lung EGFR transcript expression and observed EGFR mRNA was also significantly increased in CLAD vs. donor lung tissue (3.20-fold ± 0.70 vs. 1.28 ± 0.12, p=0.015) (Figure 2b).
Figure 2.
Whole lung transcripts for amphiregulin and EGFR are significantly upregulated in lung tissue from patients with advanced CLAD undergoing re-transplantation (n=17) as compared to normal donor lung tissue (n=12).
Areg = amphiregulin; CLAD = chronic lung allograft dysfunction
3.3. Amphiregulin and EGFR Transcript Localize to Bronchial Epithelial Cells Within OB Airways
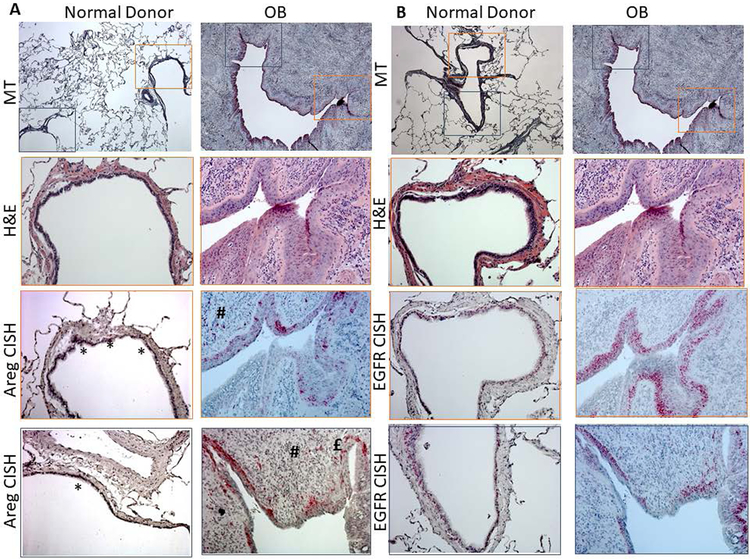
We next examined the cellular source of Areg induction within CLAD lung tissue using in situ hybridization. Representative images from two airways within one CLAD explant and one donor lung are shown in Figure 3a with additional representative images in Figure S2. Within the donor lung, Areg transcript was minimally detected. That detected localized to airway epithelial cells and appeared highly regionally focal. Within CLAD tissue, Areg transcript was abundantly detected with Areg expression predominantly localizing to airway epithelium overlying regions of intraluminal fibrosis in airways affected by OB. As opposed to the focal expression observed in donor airways, in OB affected airways Areg transcript was expressed diffusely in airway epithelial cells in a basal cell distribution. While Areg expression in CLAD tissue appeared most abundant in airway epithelium of OB lesions, we did observe consistent focal Areg transcript expression in areas corresponding with peribronchiolar inflammatory infiltrate (Figure 3a, # symbol) and occasionally in cells resembling fibroblasts in the airway smooth muscle bundles or in OB matrix (Figure S2). Similar to Areg, abundant EGFR expression was noted in the airway epithelium of OB affected airways in a pattern suggesting basal cell expansion (Figure 3b, Figure S3)
Figure 3.
Two representative airways from one normal donor and one explant CLAD lung demonstrating obliterative bronchiolitis (OB). A) Tissues are stained with Masson’s trichrome (top row, 5x), H&E (second row, 20x) or analyzed by chromogenic in situ hybridization for amphiregulin (Areg) transcript expression. (bottom two rows, 20x). Magnified areas are shown in orange and blue to demonstrate the airway epithelium. Areg transcript is only focally present in the normal donor airway epithelium (*). In contrast, in OB airway epithelium, Areg transcript is abundantly present and predominantly basally distributed. Areg transcript can also been seen in regions of infiltrating immune cells (#). B) Tissues are stained with Masson’s trichrome (top row, 5x), H&E (second row, 20x) or analyzed by chromogenic in situ hybridization for EGFR transcript expression. (bottom two rows, 20x). Magnified areas are shown in orange and blue to demonstrate the airway epithelium. Similar to Areg, abundant EGFR expression is noted in the airway epithelium of OB affected airways in a pattern suggesting basal cell expansion.
3.4. Amphiregulin Contributes to Epithelial-Derived Matrix Production in an EGFR Dependent Manner
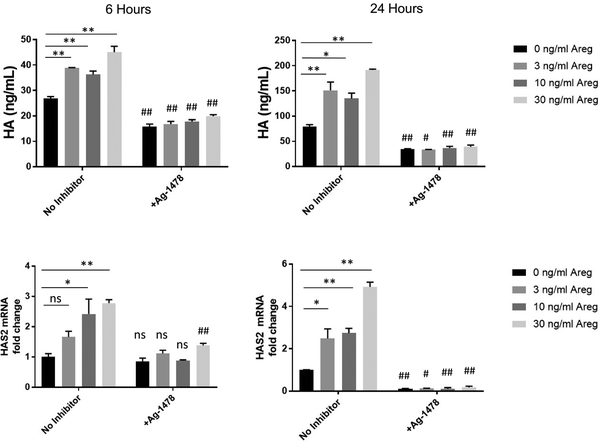
In view of these results suggesting both Areg and EGFR are induced in OB airway epithelium, we hypothesized Areg may alter epithelial cell function to induce profibrotic responses. To test this hypothesis we exposed NCI-H292 bronchial epithelial cells to Areg at increasing concentrations for 6 or 24 hours and measured production of the ECM constituent HA by ELISA and expression of the predominant HA synthase (HAS2) by RT-PCR. Our results demonstrate epithelial cells exposed to Areg significantly increase HA production and HAS2 mRNA (Figure 4). This effect was attenuated in the presence of the EGFR inhibitor AG-1478 (Figure 4) suggesting epithelial derived ECM production in response to Areg is dependent on EGFR signaling.
Figure 4.
NCI-H292 cells were stimulated with increasing concentrations of amphiregulin (Areg) for 6 or 24 hours in the absence or presence of AG-1478 with analysis for hyaluronan (HA) in the supernatant by ELSIA (a-b) or hyaluronan synthase isozyme 2 (HAS2) by RT-PCR(c-d). Epithelial cells exposed to Areg demonstrated induction of HA and mRNA for HAS2. This induction was significantly attenuated by the epidermal growth factor inhibitor AG-1478. The dose response at each time point in the absence of inhibitor was analyzed using one way ANOVA with Bonferroni correction for multiple comparisons to compare untreated to Areg treated cells at each dose (** p<0.01, *p<0.05, ns=not significant). Unpaired t-test with correction for unequal variance as appropriate was used to compare AG-1478 vs untreated cells (## p<0.01, #p<0.05, ns=not significant).
4. DISCUSSION
Although Areg is increasingly recognized to play a role in airway injury, repair, and fibrosis7,8,11,16 its contribution to CLAD in human lung transplant recipients had not been previously characterized. In this study, we analyzed two independent cohorts of lung recipients to demonstrate Areg protein is increased in the BAL in association with CLAD. Moreover, both Areg and EGFR transcript expression were increased in whole lung tissue from CLAD explant lungs. Our studies examining in situ expression of Areg transcript in explanted CLAD lungs identified bronchial epithelial cells in OB affected airways as the predominant source of Areg. Moreover, we make the novel observation that airway epithelial cells exposed to Areg alter expression of the ECM glycosaminoglycan HA in an EGFR dependent manner.
Similar to prior work, we found Areg to be barely detectable in normal human airway epithelium17. While there are no previous studies examining Areg expression in the context of CLAD or OB in human lung recipients, our observation demonstrating Areg expression localizes to the bronchial epithelium of OB affected airways is consistent those recently shown in a rodent toxin-induced OB model11. Additionally, this finding is consistent with clinical studies of non-transplant related airways diseases. For example, sputum Areg protein has been shown to be increased in patients with asthma and is negatively correlated with pulmonary function in this context16,18,19. Areg is also highly expressed in abnormal respiratory epithelium in smokers with chronic obstructive pulmonary disease17.
Our results suggest Areg is the most predominant EGFR ligand in the BAL of lung recipients with CLAD and both Areg and EGFR mRNA are highly expressed in OB affected airways. These observations have important implications in that Areg has been shown to induce a unique pattern of EGFR activation not observed with other, higher affinity EGFR ligands. Specifically, prior work has demonstrated Areg-induced EGFR activation in cultured human airway epithelial cells is associated with EGFR stabilization on the cell surface; a finding not observed with EGF-induced activation, which was associated with a reduction in total EGFR17. This same group also demonstrated Areg can promote its own expression, thus creating a positive feedback loop17.
We noted Areg expression in OB affected airway epithelium was predominantly oriented in a basal distribution. Interestingly recent work examining human airway basal cells demonstrated these cells take on altered growth and morphologic characteristics upon exposure to Areg. In particular, Areg exposed airway basal cells demonstrated increased proliferative capacity, mucous hyperplasia, and altered ciliated cell differentiation17. Thus, endogenously produced Areg may be important in expanding the basal cell population to promote airway repair in the setting of injury17. Consistent with this idea, Snyder and colleagues, observed a transient increase in whole lung Areg mRNA in an experimental model of airway injury that resolves with normal repair20. In contrast, the authors demonstrated Areg is chronically elevated in the setting of defective epithelial repair and in this context is accompanied by increased expression of ECM molecules20.
We previously reported the ECM constituent HA is increased in the BAL and blood of lung transplant recipients with CLAD and further demonstrated HA accumulates within small airways affected by OB in the lung allograft5. We now make the novel observation that NCI-H292 cells exposed to Areg increase expression of HA and its synthetic enzyme HAS2 in an EGFR dependent manner. While the effects of epithelial cell derived HA in this context are uncertain, HA has been shown to regulate innate inflammatory responses21,22, and in its low molecular weight form promotes acute lung rejection and stimulates the ingress of neutrophils, a key cell type observed in lung recipients with OB23. HA matrices can also undergo molecular modification with heavy chains from inter-a-inhibitor. Such modification of the HA matrix has been demonstrated to promote leukocyte adhesion, thus contributing to local immune activation, and is known to enhance matrix stiffness which can further amplify ECM synthesis by lung fibroblasts24–27. Moreover, prior studies have implicated HA in promoting mesenchymal progenitor cell migration and enhancing invasive cell properties28,29.
Though our study points to the airway epithelium as the most abundant cell type contributing to Areg expression in the context of OB, we also observed Areg expression in many infiltrating inflammatory cells. Current data suggests Areg can be expressed by multiple populations of activated immune cells and can also influence immune cell function30. Moreover, a recent study by Minutti et al8 in an experimental lung injury model demonstrated Areg secreted by tissue-resident immune cells is critical to regulating local concentrations of activated transforming growth factor beta (TGF-β), a key fibrotic mediator. Thus, suggesting a potential link between EGFR signaling and TGF-β mediated fibrosis in chronic inflammatory conditions. Our observations therefore, in context of this existing literature, provide a key starting point for further translational studies to delineate which immune cells contribute to Areg expression in CLAD and determine their relevance to allograft fibrosis.
While our study is the first to examine Areg in the context of CLAD and OB after lung transplantation, employed a highly translational design, and included two independent lung transplant cohorts, we acknowledge inherent limitations. First, variability in the sensitivity of the Areg protein assays employed may have contributed to diminished quantitative coverage in the UCLA cohort in particular. This notwithstanding, we demonstrated a consistent association between BAL Areg and CLAD in both clinical cohorts lending confidence to these findings. Second, we acknowledge the study of end-stage CLAD in explanted lungs may not necessarily reflect biological manifestations present at CLAD onset. Additionally, our tissue cohort for whole lung transcript analysis did not include enough R-CLAD subjects to make a determination of Areg or EGFR expression in this context. As such, we did not localize Areg expression R-CLAD explant tissue. The results of our studies provide a compelling rationale for further investigations of Areg in preclinical lung transplant models to better define the mechanistic function of Areg in OB pathogenesis. In particular, such studies will be necessary to elucidate the impact of Areg on the multifaceted immune response in the lung allograft.
In conclusion, our results implicate a novel role for Areg in lung transplant OB. Taken together our findings emphasize the importance of the epithelial response in contributing to the initiation and potentiation of airway remodeling in CLAD. When placed in the context of prior literature, our data suggest repeated airway epithelial injury in the lung allograft may induce a positive feedback loop resulting in persistent Areg overexpression, sustained EGFR activation, basal cell expansion and progressive alteration in epithelial cell function. In particular, our observations suggest EGFR dependent HA expression by airway epithelium may be a factor in the initiation of pro-fibrotic processes in CLAD. Further in-depth studies to evaluate the role of EGFR signaling in OB pathogenesis may suggest novel therapeutic targets. Ultimately, advances in understanding the mechanisms underlying normal and aberrant epithelial repair after lung allograft injury will be necessary to develop effective therapies that significantly improve lung recipient outcomes.
Supplementary Material
Acknowledgments
The authors acknowledge and thank the BioRepository & Precision Pathology Center (BRPC), a shared resource of the Duke University School of Medicine and Duke Cancer Institute for providng access to human biospecimens used under Institutional Review Board oversight in this work. The BRPC receives support from the P30 Cancer Center Support Grant (P30 CA014236). Jamie Todd was supported by an NIH K23 grant (K23AI125670). John Belperio and Sam Weigt were supported by NIH P01-HL108793.
Abbreviations:
- Areg
amphiregulin
- BAL
bronchoalveolar lavage
- BOS
bronchiolitis obliterans syndrome
- CISH
chromogenic in situ hybridization
- CLAD
chronic lung allograft dysfunction
- DUMC
Duke University Medical Center
- ECM
extracellular matrix
- EGFR
epidermal growth factor receptor
- HA
hyaluronan
- HAS2
hyaluronan synthase 2
- OB
obliterative bronchiolitis
- R-CLAD
Restrictive chronic lung allograft dysfunction
- TGF-β
transforming growth factor-β
- UCLA
University of California Los Angeles
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting Information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Christie JD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: 29th Adult Lung and Heart-Lung Transplant Report—2012. J Heart Lung Transplant. 2012;31(10):1073–1086. [DOI] [PubMed] [Google Scholar]
- 2.Ofek E, Sato M, Saito T, et al. Restrictive allograft syndrome post lung transplantation is characterized by pleuroparenchymal fibroelastosis. Mod Pathol. 2013;26(3):350–356. [DOI] [PubMed] [Google Scholar]
- 3.von der Thusen JH, Vandermeulen E, Vos R, Weynand B, Verbeken EK, Verleden SE. The histomorphological spectrum of restrictive chronic lung allograft dysfunction and implications for prognosis. Mod Pathol. 2018;31(5):780–790. [DOI] [PubMed] [Google Scholar]
- 4.Todd JL, Palmer SM. Bronchiolitis obliterans syndrome: the final frontier for lung transplantation. Chest. 2011;140(2):502–508. [DOI] [PubMed] [Google Scholar]
- 5.Todd JL, Wang X, Sugimoto S, et al. Hyaluronan contributes to bronchiolitis obliterans syndrome and stimulates lung allograft rejection through activation of innate immunity. Am J Respir Crit Care Med. 2014;189(5):556–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perugorria MJ, Latasa MU, Nicou A, et al. The epidermal growth factor receptor ligand amphiregulin participates in the development of mouse liver fibrosis. Hepatology. 2008;48(4):1251–1261. [DOI] [PubMed] [Google Scholar]
- 7.Ding L, Liu T, Wu Z, et al. Bone Marrow CD11c+ Cell-Derived Amphiregulin Promotes Pulmonary Fibrosis. J Immunol. 2016;197(1):303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minutti CM, Modak RV, Macdonald F, et al. A Macrophage-Pericyte Axis Directs Tissue Restoration via Amphiregulin-Induced Transforming Growth Factor Beta Activation. Immunity. 2019;50(3):645–654 e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blanchet S, Ramgolam K, Baulig A, Marano F, Baeza-Squiban A. Fine particulate matter induces amphiregulin secretion by bronchial epithelial cells. Am J Respir Cell Mol Biol. 2004;30(4):421–427. [DOI] [PubMed] [Google Scholar]
- 10.Lemjabbar H, Li D, Gallup M, Sidhu S, Drori E, Basbaum C. Tobacco smoke-induced lung cell proliferation mediated by tumor necrosis factor alpha-converting enzyme and amphiregulin. J Biol Chem. 2003;278(28):26202–26207. [DOI] [PubMed] [Google Scholar]
- 11.Kelly FL, Sun J, Fischer BM, et al. Diacetyl induces amphiregulin shedding in pulmonary epithelial cells and in experimental bronchiolitis obliterans. Am J Respir Cell Mol Biol. 2014;51(4):568–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly FL, Kennedy VE, Jain R, et al. Epithelial clara cell injury occurs in bronchiolitis obliterans syndrome after human lung transplantation. Am J Transplant. 2012;12(11):3076–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weigt SS, Elashoff RM, Keane MP, et al. Altered levels of CC chemokines during pulmonary CMV predict BOS and mortality post-lung transplantation. Am J Transplant. 2008;8(7):1512–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Estenne M, Maurer JR, Boehler A, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21(3):297–310. [DOI] [PubMed] [Google Scholar]
- 15.Todd JL, Jain R, Pavlisko EN, et al. Impact of forced vital capacity loss on survival after the onset of chronic lung allograft dysfunction.Am J Respir Crit Care Med. 2014;189(2):159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Habibovic A, Hristova M, Heppner DE, et al. DUOX1 mediates persistent epithelial EGFR activation, mucous cell metaplasia, and airway remodeling during allergic asthma. JCI Insight. 2016;1(18):e88811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zuo WL, Yang J, Gomi K, Chao I, Crystal RG, Shaykhiev R. EGF-Amphiregulin Interplay in Airway Stem/Progenitor Cells Links the Pathogenesis of Smoking-Induced Lesions in the Human Airway Epithelium. Stem Cells. 2017;35(3):824–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim KW, Jee HM, Park YH, Choi BS, Sohn MH, Kim KE. Relationship between amphiregulin and airway inflammation in children with asthma and eosinophilic bronchitis. Chest. 2009;136(3):805–810. [DOI] [PubMed] [Google Scholar]
- 19.Enomoto Y, Orihara K, Takamasu T, et al. Tissue remodeling induced by hypersecreted epidermal growth factor and amphiregulin in the airway after an acute asthma attack. J Allergy Clin Immunol. 2009;124(5):913–920 e911–917. [DOI] [PubMed] [Google Scholar]
- 20.Snyder JC, Zemke AC, Stripp BR. Reparative capacity of airway epithelium impacts deposition and remodeling of extracellular matrix. Am J Respir Cell Mol Biol. 2009;40(6):633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang D, Liang J, Fan J, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11(11):1173–1179. [DOI] [PubMed] [Google Scholar]
- 22.Noble PW, McKee CM, Cowman M, Shin HS. Hyaluronan fragments activate an NF-kappa B/I-kappa B alpha autoregulatory loop in murine macrophages. J Exp Med. 1996;183(5):2373–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kennedy VE, Todd JL, Palmer SM. Bronchoalveolar lavage as a tool to predict, diagnose and understand bronchiolitis obliterans syndrome. Am J Transplant. 2013;13(3):552–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu F, Mih JD, Shea BS, et al. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol. 2010;190(4):693–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swaidani S, Cheng G, Lauer ME, et al. TSG-6 protein is crucial for the development of pulmonary hyaluronan deposition, eosinophilia, and airway hyperresponsiveness in a murine model of asthma. J Biol Chem. 2013;288(1):412–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lauer ME, Aytekin M, Comhair SA, et al. Modification of hyaluronan by heavy chains of inter-alpha-inhibitor in idiopathic pulmonary arterial hypertension. J Biol Chem. 2014;289(10):6791–6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garantziotis S, Zudaire E, Trempus CS, et al. Serum inter-alpha-trypsin inhibitor and matrix hyaluronan promote angiogenesis in fibrotic lung injury. Am J Respir Crit Care Med 2008;178(9):939–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu H, Mitsuhashi N, Klein A, et al. The role of the hyaluronan receptor CD44 in mesenchymal stem cell migration in the extracellular matrix. Stem Cells. 2006;24(4):928–935. [DOI] [PubMed] [Google Scholar]
- 29.Shigeeda W, Shibazaki M, Yasuhira S, et al. Hyaluronic acid enhances cell migration and invasion via the YAP1/TAZ-RHAMM axis in malignant pleural mesothelioma. Oncotarget. 2017;8(55):93729–93740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaiss DMW, Gause WC, Osborne LC, Artis D. Emerging functions of amphiregulin in orchestrating immunity, inflammation, and tissue repair. Immunity. 2015;42(2):216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.