Figure 5.
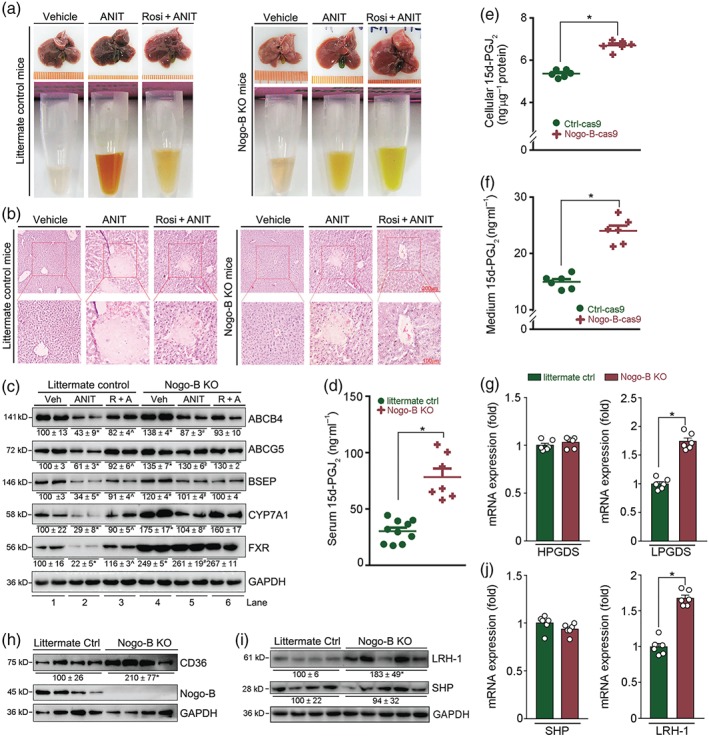
Nogo‐B deficiency protects mice against ANIT‐induced intrahepatic cholestasis by activating 15d‐PGJ2 production. (a, b) Nogo‐B KO or littermate control mice were randomly divided into three groups and given corn oil (Vehicle) by intragastric administration, ANIT (80 mg·kg−1) or ANIT plus rosiglitazone (Rosi, 30 mg·day−1·kg−1), as shown in Figure 1a. After 48 hr of ANIT treatment, mouse tissue samples were collected. Liver and serum samples were photographed (a), and (b) liver paraffin sections stained with H&E. (c) Expression of proteins (ABCB4, ABCG5, BSEP, CYP7A1, FXR, and PPARγ) extracted from littermate and Nogo‐B KO mouse liver. * P < .05, Lane 2 or 4 * P < .05, significantly different from Lane 1; ^,# P < .05, Lane 3 or 5 significantly different from Lane 2. (d) Levels of 15d‐PGJ2 in serum samples from littermate controls and Nogo‐B KO mice; all are males, 18 ± 1 g, 8 ± 0.5 weeks, n = 10 for control mice, n = 7 for Nogo‐B KO mice. * P < .05, significantly different as indicated. (e, f) Both cellular lysate and 24‐hr serum‐free conditioned medium were collected from Ctrl‐cas9 and Nogo‐B‐cas9 cells at the same density (~80% confluence). Levels of 15d‐PGJ2 in cellular lysate (e), and conditioned medium (f) were determined using an ELISA assay kit. Cellular protein content was determined and used to normalize cellular 15d‐PGJ2 content (ng·μg−1 protein, f). * P < .05, significantly different as indicated; (n = 6). (g–j) Total cellular proteins or RNA were extracted from liver samples of littermate control and Nogo‐B KO mice. Expression of haematopoietic PGDS (HPGDS) and lipocalin PGDS (LPGDS) mRNA (g), CD36 protein (h), LRH‐1, and SHP protein (i) or mRNA (j) was determined by Western blot or qRT‐PCR. * P < .05, significantly different as indicated; (n = 6)
