Abstract
Background
Clinically evident cardiac involvement has been documented in 5% of sarcoidosis patients, primarily manifesting as heart block, ventricular arrhythmias, and heart failure. Heart Rhythm Society consensus guidelines recommend advanced cardiac imaging with fluorodeoxyglucose–positron emission tomography (FDG-PET) scan for diagnosis of cardiac sarcoidosis, given endomyocardial biopsy’s low sensitivity.
Case summary
We describe four patients with cardiac sarcoidosis diagnosed with FDG-PET scan performed using a standardized imaging protocol for cardiac sarcoidosis. Serial FDG-PET scans were performed to monitor disease progression and response to therapy. Patients 1 and 2 presented with heart block, Patient 3 with heart failure and ventricular tachycardia (VT), and Patient 4 with VT. Patient 1 showed an initial decrease in standard uptake value (SUV) on immunosuppression, followed by an increase in SUV, necessitating steroid therapy. Patient 2’s SUV decreased on immunosuppression. Patient 3 required 3.5 years of immunosuppression for the SUV to decrease to inactive disease levels, with SUV increasing and decreasing at different times during treatment, and subsequently developed VT. For Patient 4, areas of inflammation on the initial scan matched low voltage areas on the patient’s EP study, confirming the arrhythmia’s pathophysiological basis.
Discussion
Cardiac sarcoidosis progression and response to therapy are heterogeneous. Serial FDG-PET scans are useful to diagnose disease, tailor therapy, and monitor the clinical course of disease, allowing treatment decisions to be based on the quantitative level of inflammation seen on FDG-PET.
Keywords: Cardiac sarcoidosis, Arrhythmias, Heart block, Ventricular tachycardia, Heart failure, FDG-PET, Case series
Learning points
Cardiac sarcoidosis can follow a heterogeneous and unpredictable clinical course, making response monitoring with follow-up scans a priority.
Fluorodeoxyglucose–positron emission tomography (FDG-PET) scans are useful to diagnose disease, tailor therapy, and monitor the clinical course of disease, allowing treatment decisions to be based on the quantitative level of inflammation seen on FDG-PET.
Areas of inflammation on PET scan correlate with regions with low voltage on EP study mapping, as well as on pathological examination.
Introduction
Cardiac sarcoidosis is a granulomatous inflammatory disease thought to be caused by a combination of immunologic, genetic, and environmental factors1,2 and commonly manifests as conduction abnormalities, ventricular arrhythmias, heart failure, and sudden cardiac death.2,3 Early detection of cardiac involvement enables initiation of immunosuppressive and/or cardiac device therapy.4,5 The Heart Rhythm Society consensus diagnostic guidelines for cardiac sarcoidosis recommend advanced cardiac imaging with cardiac fluorodeoxyglucose–positron emission tomography (FDG-PET) or cardiac magnetic resonance imaging.1,6 Fluorodeoxyglucose–positron emission tomography has been shown to be more sensitive6–8 and can monitor response to treatment by relative change in FDG avidity of a region with active sarcoidosis, corresponding to a relative change in inflammation.2,9
Few reports have explored the use of cardiac FDG-PET scans to track disease progression or tailor treatment with immunosuppressive therapy. Therefore, this case series presents four patients with varying manifestations of cardiac FDG-PET confirmed cardiac sarcoidosis, three with documented post-treatment serial follow-up scans, and one with direct pathological confirmation of cardiac sarcoidosis correlating with inflammation noted on FDG-PET scanning.
Timeline
| Patient 1 | |
| T = 0 | Initial scan |
| 12 months | Scan 2 |
| 20 months | Scan 3 |
| 26 months | Scan 4 |
| Patient 2 | |
| T = 0 | Initial scan |
| 6 months | Scan 2 |
| Patient 3 | |
| T = 0 | Initial scan |
| 5 months | Scan 2 |
| 9 months | Scan 3 |
| 15 months | Scan 4 |
| 22 months | Scan 5 |
| 27 months | Scan 6 |
| 34 months | Scan 7 |
| 38 months | Scan 8 |
| Patient 4 | |
| T = 0 | Initial scan |
| 10 months | Cardiac transplant |
Methods
The study protocol was approved by the Columbia University Irving Medical Center (CUIMC) Institutional Review Board. All patients included in this case series were seen at the CUIMC programme in cardiac electrophysiology between 2008 and 2018. All scans included in this series were performed using the standardized imaging protocol for cardiac sarcoidosis proposed by the Cardiovascular Council of the Society of Nuclear Medicine and Molecular Imaging.10 The patients underwent an 18–24-h carbohydrates fast and were nil per os (NPO) for at least 6 h before the scan. Rest perfusion was obtained with 13N-Ammonia first. Heparin was administered intravenously 15 min prior to the FDG scan (10–50 IU/kg) unless clinically advised against. Inflammation scans were performed 90 min after 18F-FDG injection.
Briefly, interpretation of cardiac FDG-PET scans uses regional comparison of the myocardium’s perfusion to its uptake of FDG9,11,12 which is related to the metabolic activity of stimulated cells and disease activity. Activated macrophages that form the pathognomonic sarcoid granulomas have increased FDG uptake visible on the PET scan, quantified by the standard uptake value (SUV).9,13,14 Standard uptake value greater than 2.6 with heparin or 2.3 without heparin is considered active disease.10 Mean absolute myocardial blood flow (MBF) in mL/g/min at rest and % scarred myocardium (of total left ventricular volume) are also reported. Timing of FDG-PET scanning was performed at the discretion of the treating physician based on clinical course, including response to treatment.
Case presentation
Complete heart block
Patient 1
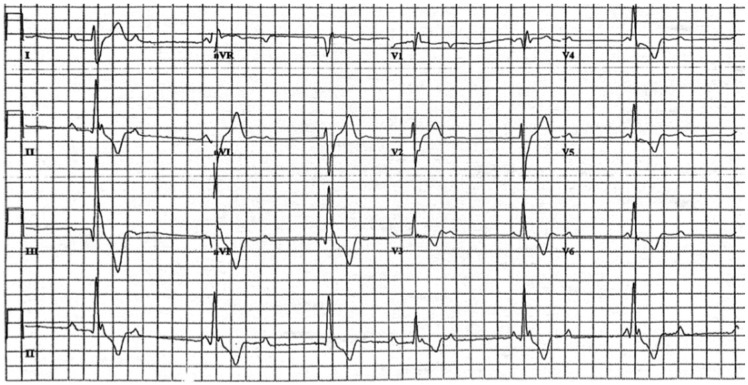
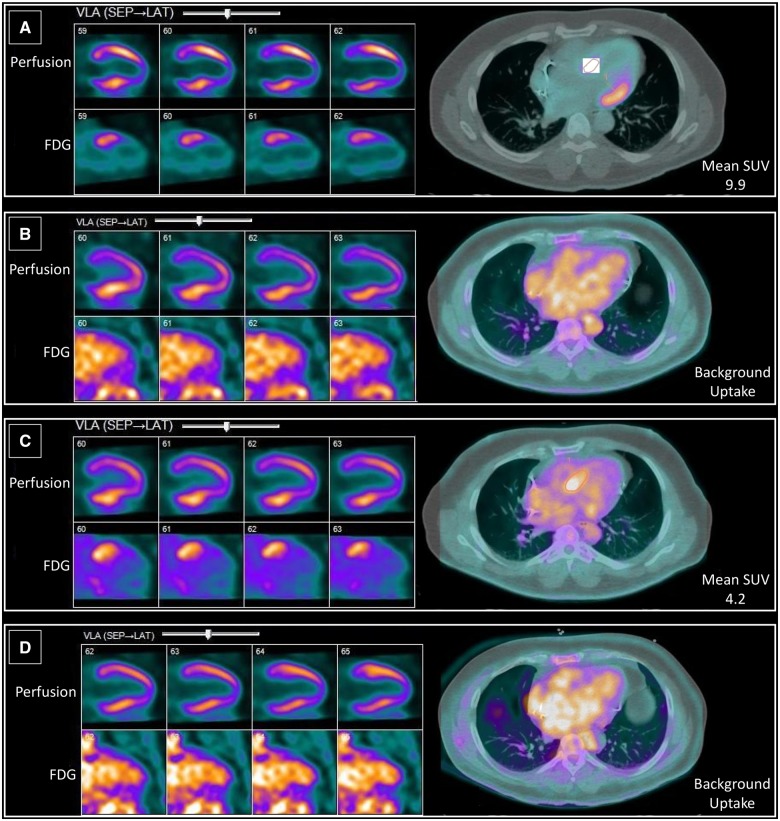
A 56-year-old man presented to an outside institution with light-headedness. He was found to be in high-grade atrioventricular (AV) block with a ventricular escape rate of ∼40 b.p.m. (Figure 1). He had a reported history of pulmonary sarcoidosis and was not on immunosuppressive therapy. No clear cause for the heart block was identified, and a pacemaker was implanted. He later presented to CUIMC with progressive dyspnoea on exertion. His electrocardiogram noted an atrial-sensed, ventricle-paced rhythm. Echocardiogram (ECHO) revealed a left ventricular ejection fraction (LVEF) of 35%, dilated left ventricle, and moderate global hypokinesis. He underwent a cardiac FDG-PET scan which revealed decreased perfusion and increased FDG metabolism in the basal anterior and basal antero-lateral walls, consistent with cardiac sarcoidosis (Figure 2A).
Figure 1.
Electrocardiogram from Patient 1 noting sinus rhythm with high-grade atrioventricular block and wide escape rhythm (with one probable narrower, conducted complex). Scale = 25 mm/s, 10 mm/mV.
Figure 2.
Vertical long axis view images comparing 13N-ammonia perfusion scan (top) with 18F-fluorodeoxyglucose scan (bottom) beside fused transaxial images of 18F-fluorodeoxyglucose scan. Perfusion-metabolism mismatch is visible in the basal anterior and basal antero-lateral walls in A and C. (B and D) No active sarcoidosis is shown (Patient 1).
Follow-up
Due to the higher frequency of ventricular arrhythmias and sudden death seen in cardiac sarcoidosis, as well as the need for continuous right ventricular pacing given a reduced LVEF <50%, and guidelines noting that implantable cardioverter-defibrillator (ICD) can be utilized for cardiac sarcoidosis patients (Class IIa recommendation when LVEF <35% and IIb when LVEF 36–49%), the patient’s pacemaker was upgraded to a biventricular ICD.1,15 He was started on 60 mg of prednisone, which was not well tolerated, then initiated on methotrexate. He had three follow-up scans over the subsequent 26 months (Table1). A repeat scan revealed decreased perfusion and no focal myocardial FDG uptake, consistent with no active cardiac sarcoidosis (Figure 2B). The third scan (off immunosuppressants) showed perfusion-metabolism mismatch, suggestive of active cardiac sarcoidosis (Figure 2C). A fourth scan on 20 mg prednisone revealed no active sarcoidosis (Figure 2D). Brain natriuretic peptide (BNP) level, which at diagnosis was elevated at 282 pg/mL (normal < 177), decreased to within the normal range (46.9 pg/mL) at last follow-up.
Table 1.
Patient 1’s serial fluorodeoxyglucose–positron emission tomography scans
| Scan number | Months post-initial scan | Immunosuppressant | SUV | LVEF (%) | MBF (mL/g/min) | % Scarring |
|---|---|---|---|---|---|---|
| 1 (initial) | 0 | — | 9.9 | 35 | 1.12 | 0% |
| 2 | 12 | Methotrexate | — | 52 | 1.08 | 0% |
| 3 | 20 | — | 4.2 | 48 | 0.91 | 0% |
| 4 | 26 | 20 mg prednisone | — | 54 | 1.05 | 0% |
Patient 2
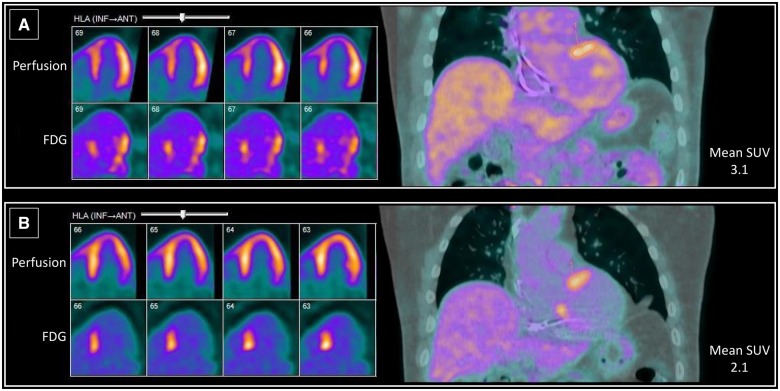
A 55-year-old man experienced three episodes of syncope before being found to be incomplete heart block on a Holter monitor. A permanent pacemaker was implanted after potential reversible causes, including Lyme disease, were excluded. His ECHO noted reduced LVEF of 20–25% in the setting of continuous right ventricular pacing. Cardiac FDG-PET scanning revealed elevated FDG uptake in the anterobasal, anterior, and inferoseptal regions in the absence of myocardial scar (Figure 3A) with a SUV of 3.1, LVEF of 33%, and left ventricular end diastolic volume (LVEDV) of 156 mL. Rest MBF = 0.56 mL/g/min and scar = 0%.
Figure 3.
Baseline (A) and follow-up (B) horizontal long-axis view images comparing 13N-ammonia perfusion scan with 18F-fluorodeoxyglucose scan beside fused coronal images of 18F-fluorodeoxyglucose scan (Patient 2).
Follow-up
His pacemaker was upgraded to a biventricular ICD and he was started on 60 mg of prednisone with a 6-month taper. Follow-up FDG-PET at 6 months (Figure 3B) demonstrated normal perfusion and a smaller area of FDG uptake than the initial scan, with a decreased SUV (2.1). Rest MBF = 0.63 mL/g/min and scar = 0%. The area of FDG uptake on the follow-up scan was localized to the septum. The patient has been maintained on 5 mg prednisone with an increase in his LVEF on ECHO from 20–25% to 40% nearly 2 years after his diagnosis of cardiac sarcoidosis. Cardiac BNP, which was initially elevated at 608 pg/mL at diagnosis, was within normal range after 1 year of follow-up.
Acute decompensated heart failure and ventricular tachycardia
Patient 3
A 50-year-old man with a 5-year history of pulmonary sarcoidosis and ventricular tachycardia (VT) requiring ICD was transferred for acute shortness of breath and orthopnoea due to a new diagnosis of acute decompensated heart failure. He received dobutamine-assisted diuresis and advanced circulatory support with an intra-aortic balloon pump. An endomyocardial biopsy showed no inflammatory infiltrates or granulomas. Despite the unrevealing biopsy, cardiac FDG-PET scanning demonstrated decreased perfusion at the anteroapical, mid-anterior, anterolateral, and mid-inferolateral walls, with increased FDG uptake at the anteroapical, mid-anterior, apical lateral, and mid-lateral walls, consistent with cardiac sarcoidosis. The SUV was 5.0, rest MBF = 0.56 mL/g/min, and scar = 6%. Left ventricular ejection fraction was 20% with left ventricular dilation and akinesis of the apical anterior, apical lateral, apical inferior, and apical septal walls. Initial BNP was elevated at 2208 pg/mL.
Follow-up
The patient was started on 60 mg of prednisone and followed with seven additional PET scans over a period of nearly 4 years (Table2). While his last scan revealed resolution of active inflammatory cardiac sarcoidosis, scarring was increased. He was shortly thereafter admitted for an ICD discharge, with interrogation noting monomorphic VT. An EP study revealed three different foci for VT. Endocardial ablation was performed in multiple areas of scar, but the foci were felt to be epicardial in origin, and epicardial ablation was deferred due to diffuse disease and haemodynamic instability during the procedure. He soon thereafter developed mixed cardiogenic and septic shock due to a lower extremity wound infection and died.
Table 2.
Patient 3’s serial fluorodeoxyglucose–positron emission tomography scans
| Scan number | Months post-initial scan | Prednisone (mg daily) | Mycophenolate mofetil (mg twice daily) | SUV | LVEF (%) | MBF (mL/g/min) | % Scarring |
|---|---|---|---|---|---|---|---|
| 1 (initial) | 0 | — | — | 5.0 | 20 | 0.56 | 6% |
| 2 | 5 | 60 | — | 3.4 | 17 | 0.57 | 6% |
| 3 | 9 | 60 | — | 2.5 | 25 | 0.57 | 12% |
| 4 | 15 | 25 | 1500 | 2.7 | 29 | 0.58 | 12% |
| 5 | 22 | 15 | 1500 | 6.4 | 19 | 0.58 | 24% |
| 6 | 27 | 10 | 2500 | 9.0 | 26 | 0.6 | 24% |
| 7 | 34 | Unknown | Unknown | 3.0 | 31 | 0.61 | 29% |
| 8 | 38 | Unknown | Unknown | 1.8 | Unknown | Unknown | Unknown |
Patient 4
A 48-year-old man with non-ischaemic cardiomyopathy, a prior episode of VT (status post-ICD implantation), and a family history of sarcoidosis presented with near syncope and ICD shock. An EP study at his local hospital revealed low-voltage areas along the mitral annulus, tricuspid annulus, and septum, which were ablated as part of a substrate modification strategy. He was referred for cardiac FDG-PET scanning 2 weeks post-ablation, revealing decreased perfusion and increased FDG uptake in the areas of low voltage encountered during EP study mapping (SUV = 4.8, MBF = 0.59 mL/g/min, and scar = 0%).
Follow-up
The patient was initiated on high-dose prednisone and methotrexate for immunosuppression. One month post-ablation, ICD interrogation showed no further episodes of VT. Three months post-ablation a slow taper of his steroids was initiated and he was continued on methotrexate. Ten months after the ablation the patient underwent cardiac transplantation for refractory heart failure. The case is presented because tissue pathology of the regions identified on FDG-PET (that were targeted for VT ablation) revealed multinucleated giant cells and lymphocytic infiltration, pathognomonic for sarcoidosis (Figure 4).
Figure 4.
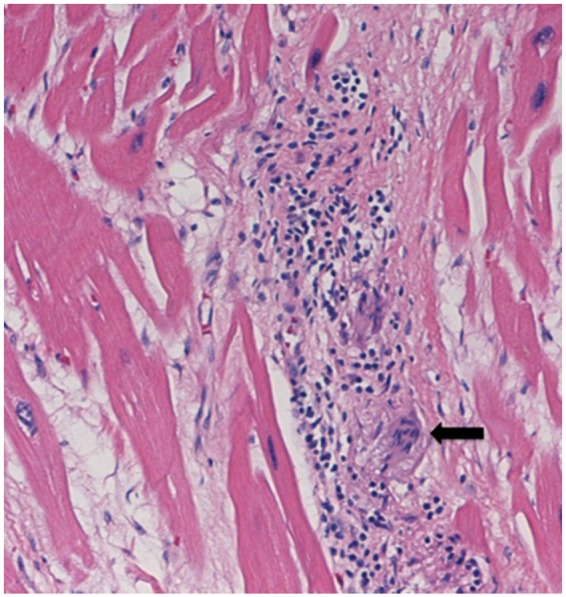
Histology sample from explanted heart (Patient 4). Black arrow indicates a multinucleated giant cell. Lymphocytic infiltration is also visible. (Printed with permission of Oxford University Press, 21 June 2019, licence number 4613750798712).
Discussion
These patients’ initial presentations of heart block, heart failure, and VT represent the three most common clinical manifestations of cardiac sarcoidosis. Follow-up scans were performed for three of the four patients, allowing clinicians to monitor physiological responses to immunosuppressive therapy. In the fourth patient, who underwent heart transplant, FDG-PET scanning was used to correlate anatomical areas and electrophysiology related complications, allowing for direct pathological correlation of cardiac sarcoidosis with prior FDG-PET scanning.
Cardiac FDG-PET details the high metabolic activity of active inflammatory lesions,11 allowing for early initiation of immunosuppressive therapy, which has been shown to halt progression of disease and improve ejection fraction and conduction abnormalities.2,16,17 While extracardiac or cardiac biopsy is required to confirm the diagnosis of cardiac sarcoidosis, patients were counselled that because endomyocardial biopsy has a relatively low yield of 50% even if performed with EP or image-guided (PET) assistance, the diagnoses, though not confirmed, were highly suspect in the setting of the clinical scenario and PET findings, and we would recommend immunosuppression regardless of biopsy result.18
Improvement in inflammation on the PET scan and other clinical measures in response to immunosuppression was heterogeneous, mirroring the literature.1,2,5 For Patient 1, inflammation initially improved on immunosuppresants as noted by a decreased SUV, then increased off immunosuppresants by the third scan, and completely resolved by the fourth scan, and LVEF followed a similar trend. Patient 2 also demonstrated active sarcoidosis on a follow-up scan despite 6 months of immunosuppression. Patient 3, presenting very late with acute decompensated heart failure, manifested an increase in LVEF on FDG-PET scan (20–31%) with immunosuppression and medical therapy for congestive heart failure. However, the response to therapy was non-linear. Over 3 years of immunosuppression were required before inactive disease was achieved, a much longer time course than has previously been reported, and supports a slow steroid taper strategy in select patients.19
The cardiac PET-FDG scans included in this series also show interesting anatomic correlations for the patients who presented with arrhythmias (i.e. heart block or VT). Patients with some degree of heart block (Patients 1 and 2) had inflammation at the anterior septum, near the AV node. Additionally, Patient 4 demonstrated a correlation between areas of inflammation on PET scan and regions with low voltage on EP study mapping, as well as on pathological examination.
Conclusion
Cardiac sarcoidosis represents a diagnostic and therapeutic challenge. The cases we reported show that the progression of disease and response to therapy is heterogeneous and that serial FDG-PET scans can be useful not only to diagnose disease but also to monitor the clinical course of disease. Thus, with the help of serial scans, treatment decisions can be informed by the presence and the quantitative level of inflammation (i.e. SUV), as opposed to relying solely on less objective measures of patients’ clinical status.
Lead author biography

Cooper B. Kersey is a first-year resident in internal medicine at the University of Washington. He graduated from Columbia University Vagelos College of Physicians and Surgeons in June 2019.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: IRB approval for retrospective analyses of patient data without patient consent was obtained for this study, as: i) the research involved a chart review that utilized confidential methods in order to ensure that collected, stored, and reported information cannot be linked to patients (e.g., through the use of patient deidentifiers and locked information storage); and ii) the research could not practicably be carried out without the waiver or alteration, as all information was collected retrospectively.
Conflict of interest: none declared.
Supplementary Material
References
- 1. Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, Judson MA, Kron J, Mehta D, Cosedis Nielsen J, Patel AR, Ohe T, Raatikainen P, Soejima K.. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm 2014;11:1304–1323. [DOI] [PubMed] [Google Scholar]
- 2. Ho JSY, Chilvers ER, Thillai M.. Cardiac sarcoidosis—an expert review for the chest physician. Expert Rev Respir Med 2018;13:507–520. [DOI] [PubMed] [Google Scholar]
- 3. Fleming HA. Sarcoid heart disease. Br Heart J 1974;36:54–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roberts WC, McAllister HA Jr, Ferrans VJ.. Sarcoidosis of the heart. A clinicopathologic study of 35 necropsy patients (group 1) and review of 78 previously described necropsy patients (group 11). Am J Med 1977;63:86–108. [DOI] [PubMed] [Google Scholar]
- 5. Sohn DW, Park JB, Lee SP, Kim HK, Kim YJ.. Viewpoints in the diagnosis and treatment of cardiac sarcoidosis: proposed modification of current guidelines. Clin Cardiol 2018;41:1386.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Orii M, Hirata K, Tanimoto T, Ota S, Shiono Y, Yamano T, Matsuo Y, Ino Y, Yamaguchi T, Kubo T, Tanaka A, Akasaka T.. Comparison of cardiac MRI and 18F-FDG positron emission tomography manifestations and regional response to corticosteroid therapy in newly diagnosed cardiac sarcoidosis with complete heart block. Heart Rhythm 2015;12:2477–2485. [DOI] [PubMed] [Google Scholar]
- 7. Youssef G, Leung E, Mylonas I, Nery P, Williams K, Wisenberg G, Gulenchyn KY, deKemp RA, DaSilva J, Birnie D, Wells GA, Beanlands RSB.. The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: a systematic review and metaanalysis including the Ontario experience. J Nucl Med 2012;53:241–248. [DOI] [PubMed] [Google Scholar]
- 8. Erath JW, Puntmann VO, Chavakis E, Hohnloser SH.. Syncope on exertion in a young male. HeartRhythm Case Rep 2018;4:324–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Akaike G, Itani M, Shah H, Ahuja J, Yilmaz Gunes B, Assaker R, Behnia F.. PET/CT in the diagnosis and workup of sarcoidosis: focus on atypical manifestations. Radiographics 2018;38:1536–1549. [DOI] [PubMed] [Google Scholar]
- 10. Chareonthaitawee P, Beanlands RS, Chen W, Dorbala S, Miller EJ, Murthy VL, Birnie DH, Chen ES, Cooper LT, Tung RH, White ES, Borges-Neto S, Di Carli MF, Gropler RJ, Ruddy TD, Schindler TH, Blankstein R.. Joint SNMMI-ASNC Expert Consensus Document on the role of (18)F-FDG PET/CT in cardiac sarcoid detection and therapy monitoring. J Nucl Med 2017;58:1341–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Okumura W, Iwasaki T, Toyama T, Iso T, Arai M, Oriuchi N, Endo K, Yokoyama T, Suzuki T, Kurabayashi M.. Usefulness of fasting 18F-FDG PET in identification of cardiac sarcoidosis. J Nucl Med 2004;45:1989–1998. [PubMed] [Google Scholar]
- 12. Bokhari S, Lin JC, Julien HM. FDG-PET is a superior tool in the diagnosis and management of cardiac sarcoidosis. American College of Cardiology website. 2017. https://www.acc.org/latest-in-cardiology/articles/2017/04/10/08/43/fdg-pet-is-a-superior-tool (26 September 2018).
- 13. Glaudemans AW, de Vries EF, Galli F, Dierckx RA, Slart RH, Signore A.. The use of 18F-FDG-PET/CT for diagnosis and treatment monitoring of inflammatory and infectious diseases. Clin Dev Immunol 2013;2013:1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee P-I, Cheng G, Alavi A.. The role of serial FDG PET for assessing therapeutic response in patients with cardiac sarcoidosis. J Nucl Cardiol 2017;24:19–28. [DOI] [PubMed] [Google Scholar]
- 15. Al-Khatib SM, Stevenson WG, Ackerman MF, Bryant WJ, Callans DJ, Curtis AB.. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Circulation 2018;138:e272–e391. [DOI] [PubMed] [Google Scholar]
- 16. Kandolin R, Lehtonen J, Airaksinen J, Vihinen T, Miettinen H, Ylitalo K, Kaikkonen K, Tuohinen S, Haataja P, Kerola T, Kokkonen J, Pelkonen M, Pietilä-Effati P, Utrianen S, Kupari M.. Cardiac sarcoidosis: epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation 2015;131:624–632. [DOI] [PubMed] [Google Scholar]
- 17. Flores RJ, Flaherty KR, Jin Z, Bokhari S.. The prognostic value of quantitating and localizing F-18 FDG uptake in cardiac sarcoidosis. J Nucl Cardiol 2019;26:288–297. [DOI] [PubMed] [Google Scholar]
- 18. Birnie DH, Nery PB, Ha AC, Beanlands RS.. Cardiac sarcoidosis. J Am Coll Cardiol 2016;68:411–421. [DOI] [PubMed] [Google Scholar]
- 19. Goh CY, Gay A, Hofman MS, Wong C, Westcott J, Better N.. Role of PET/CT in multimodality imaging in differentiating cardiac sarcoidosis from arrhythmogenic right ventricular dysplasia. J Nucl Cardiol 2018;doi: 10.1007/s12350-018-1382-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.