Abstract
Background
Fabry disease (FD) is an X-linked lysosomal storage disorder resulting from a deficiency in alpha-galactosidase A. The major causes of death due to cardiac complications include life-threatening arrhythmias. In addition, life-threatening arrhythmias may be related to myocardial fibrosis assessed by late gadolinium enhancement (LGE).
Case summary
A 43-year-old man with sinus bradycardia and left ventricular hypertrophy was referred to our cardiology department. Family history includes unexplained hypertrophy and sick sinus syndrome in mother. Additionally, his plasma alpha-galactosidase A activity was low. He was subsequently diagnosed with FD. Enzyme replacement therapy using 1.0 mg/kg agalsidase-β was initiated. During the fifth administration, he developed ventricular fibrillation (VF). Electrocardiography conducted immediately before VF revealed ST elevation in the inferior leads with reciprocated ST depression. Cardiac magnetic resonance imaging showed no LGE in the myocardium. Coronary angiography showed no organic stenosis; moreover, coronary spasms were induced by an intracoronary acetylcholine injection. Ventricular fibrillation was not observed as the patient received calcium antagonists.
Discussion
This report suggests that vasospastic angina pectoris is associated with life-threatening arrhythmias in patient with FD without LGE.
Keywords: Fabry disease, Vasospastic angina pectoris, Ventricular fibrillation, Case report
Learning points
Vasospastic angina pectoris could be associated with VF development in patients with FD without late gadolinium enhancement who experienced unexplained VF.
Spasm provocation test may be considered if patients with FD experience idiopathic VF.
Introduction
Fabry disease (FD) is an X-linked lysosomal storage disorder caused by the deficiency of alpha-galactosidase A. The intracellular storage of globotriaosylceramides (GL-3) in various tissues and organs leads to a multisystemic disease that affects the kidneys, nervous systems, and heart.1 Cardiac involvement is common in FD and manifests as ventricular hypertrophy, systolic and diastolic dysfunctions, and conduction disturbances.2 Meanwhile, some patients with FD were reported to have vasospastic angina pectoris (VSA) without organic stenosis of the coronary artery.3,4 The major causes of death due to cardiac complications in FD are life-threatening arrhythmias such as ventricular tachycardia (VT) and ventricular fibrillation (VF). In addition, myocardial fibrosis assessed by late gadolinium enhancement (LGE) using cardiac magnetic resonance imaging (MRI) was suggested to be associated with life-threatening arrhythmias in patients with FD.5
Herein, we present the case of VF associated with VSA in a patient with FD.
Timeline
| October 2016 | Diagnosis of sick sinus syndrome and apical hypertrophy |
| December 2016 | Pacemaker implantation |
| August 2017 | Diagnosis of Fabry disease |
| January 2018 | Initiation of enzyme replacement therapy (ERT) |
| March 2018 | Development of ventricular fibrillation (VF) during the administration of ERT |
| Electrocardiogram obtained just before VF showed J-point elevation in the inferior leads | |
| May 2018 | Implantation of implantable cardiac defibrillator |
| August 2018 | Diagnosis of vasospastic angina pectoris |
Case presentation
A 43-year-old man was referred to our cardiology department for the evaluation of abnormal 12-lead electrocardiogram (ECG) findings. Electrocardiogram showed sinus bradycardia, high voltage in the left precordial leads, and inverted T waves in the inferolateral leads. PR interval, QRS duration, and axis were within normal range. Despite the symptoms of dizziness, the patient had not sought a medical opinion. He suffered from meningitis at 15 years of age and could barely hear with his right ear since then. He had no history of smoking. His mother had unexplained left ventricular hypertrophy, and a pacemaker was implanted for sick sinus syndrome. She died of heart failure a few years before the patient presented to our hospital. Clinical cardiac evaluation of the patient revealed a regular heart rate of 38 b.p.m., normal heart sounds, and no murmurs. Arterial blood pressure was 102/77 mmHg. Chest X-ray showed normal cardiothoracic ratio and no pulmonary oedema. Echocardiography showed normal ventricular systolic and diastolic function (left ventricular ejection fraction: 65%, E/A ratio: 1.8) and apical hypertrophy (maximal wall thickness: 16 mm) (Figure 1). The obstruction of left ventricle and valvular abnormalities were not shown. Laboratory examination revealed almost normal findings; however, proteinuria was noted. Coronary angiography showed no organic stenosis. Holter ECG revealed sinus arrest for >5 s, which was associated with pre-syncopal symptoms. Therefore, he was diagnosed with sick sinus syndrome, for which a dual-chamber pacemaker was implanted. Based on family history, a hereditary cardiac disease was suspected. However, his plasma alpha-galactosidase A activity had decreased (0.5 nmol/h/mL) compared with the normal value of >4.0 nmol/h/mL. Endomyocardial biopsy assessed using an electron microscope revealed lamellate deposits within cardiomyocytes. Renal biopsy revealed GL-3 deposits in the endothelial cells of glomeruli. The patient also exhibited a missense mutation of GLA genes in exon 7 that leads to an arginine-to-glutamine (R342Q) missense mutation. Thus, he was diagnosed with FD. He did not have the other involvement of FD. Enzyme replacement therapy (ERT; agalsidase-β: 1.0 mg/kg: the mice recombinant form) was then initiated.
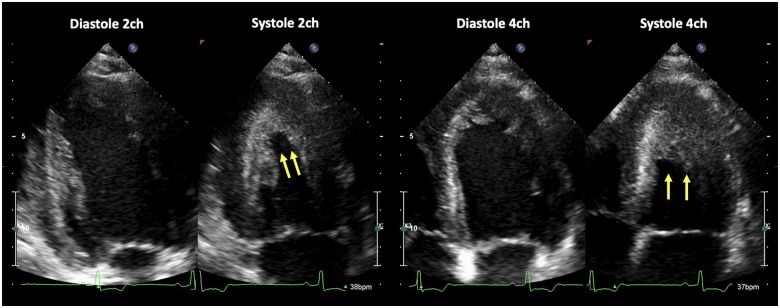
Figure 1.
Echocardiography revealed left ventricular apical hypertrophy in two- and four-chamber apical views (yellow arrows).
After the second administration of ERT, he developed a low-grade fever. After administering an antipyretic before next ERT, his fever subsided. Antibody analysis revealed an immunoglobulin-G, not immunoglobulin-E, for agalsidase-β. During the fifth administration of ERT, he suddenly experienced cardiopulmonary arrest. Electrocardiogram showed VF. Cardioversion was immediately initiated, and he was resuscitated.
Because the patient was unconscious after resuscitation, respiratory care using mechanical ventilator and target temperature management (TTM) at 35°C were performed. However, the target temperature was changed from 35°C to 36°C and was managed using percutaneous cardiopulmonary system because of the subsequent episode of VF storm. The 12-lead ECG obtained just before VF showed ST elevation in leads II, III, and aVF (Figure 2). Ventricular paced beats were increased because the mechanism of VF development was R on T in ECG monitor. He was confirmed to have no neurological sequelae after TTM, and there was an upgrade to an implantable cardiac defibrillator (ICD) for secondary prevention. Cardiac MRI, performed to assess myocardial fibrosis, revealed no LGE in the myocardium (Figure 3). He underwent ERT (0.2 mg/kg agalsidase-α: the human recombinant form) again 2 months after the first VF development. He exhibited no infusion-associated-reaction. However, VF developed at midnight and was defibrillated by ICD. Coronary angiography was performed again because the ECG obtained before VF was suspected to show ischaemic ST-T changes. The patient had no organic stenosis in the coronary artery; however, spasms were induced following intracoronary acetylcholine injection (50–100 μg) in segments 1, 4, 6, 8, and 11 of the coronary artery (Figure 4). The patient had never had chest pain before then, but diltiazem (100 mg once a day) was administered. Ventricular fibrillation did not develop since then.
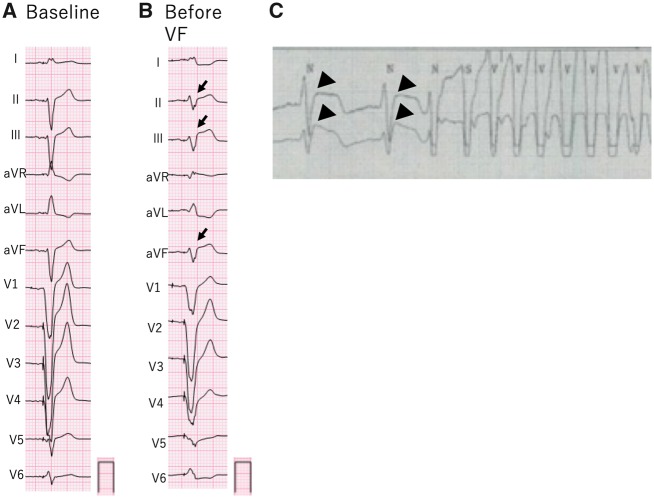
Figure 2.
(A) At baseline, electrocardiogram was obtained during right ventricular stimulation. (B) Electrocardiogram obtained just before ventricular fibrillation showed ST-elevation in the inferior leads and reciprocated ST depression in leads I, aVL, and V6 (arrows). (C) Before the onset of ventricular tachycardia, monitoring electrocardiogram showed marked ST elevation (broad arrows).
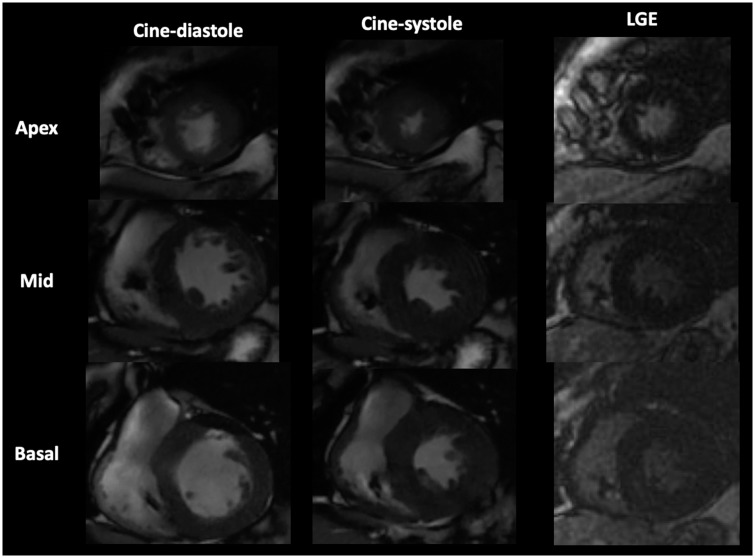
Figure 3.
Cardiac magnetic resonance imaging showed left ventricular apical hypertrophy and the absence of late gadolinium enhancement in short-axis view.
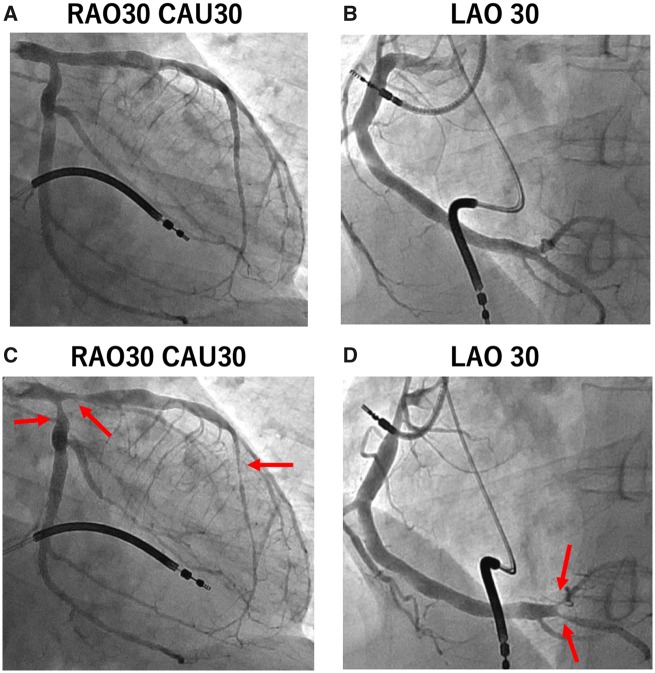
Figure 4.
(A, B) No significant coronary stenosis was found in the right and left coronary arteries in control coronary angiography. (C, D) Coronary artery spasms were induced following intracoronary acetylcholine injection in the right and left coronary arteries (red arrows).
Discussion
This case report suggests that VSA is associated with VF in patients with FD without LGE.
Patients with FD developed several arrhythmias, and most of them died owing to cardiac complications.6 Takenaka et al.7 reported ventricular arrhythmias in patients with terminal-stage cardiac FD; autopsies revealed marked fibrosis in the thinned base region of the left ventricular posterior wall. Weidemann et al.8 detected VT in patients with FD and LGE using cardiac MRI. LGE enables to identify focal myocardial fibrosis. However, recent research shows the usefulness of native T1 mapping to detect diffuse myocardial fibrosis in patients with dilated cardiomyopathy.9 In this case, native T1 mapping was not performed, and focal myocardial fibrosis was absent because LGE was not detected on cardiac MRI.
Therefore, VT, which frequently originated at the site of focal myocardial fibrosis was not associated with malignant arrhythmia in this case. ST-T changes were observed during right ventricular pacing. However, ECG obtained before VF revealed ST elevation in the inferior leads and reciprocated ST depression in leads I, aVL, and V6 compared with those obtained through ECG of baseline observed during right ventricular pacing and were believed to reveal myocardial ischaemia. Transmural voltage gradients between the epicardium and endocardium caused by this myocardial ischaemia owing to VSA were believed to increase ventricular vulnerability and cause VF development. Cooling during TTM might be a provocative factor of coronary vasospasm and result in VF storm. The patient had immunoglobulin-G for an agalsidase-β. An allergic reaction could not induce VF because VF had developed when agalsidase-β was not administered, and the ICD had once defibrillated after restarting ERT with agalsidase-α for which the patient showed no allergic reaction. Moreover, the presence of immunoglobulin-E for agalsidase-β, associated with anaphylactic shock, was not reported.
Some patients with FD without organic stenosis of the coronary artery have angina.2 Chimenti et al.10 suggested the significance of narrowed intramural coronary arteries owing to hypertrophy in patients with FD accompanied by angina without organic stenosis on coronary angiography. Some patients with FD have VSA because GL-3 deposits in the endothelial cells of myocardial capillaries may induce excessive vasoconstriction.
In this report, although the patient had FD without LGE, he had VSA, which could be associated with VF. These findings suggest the importance of examining VSA in patients with FD without LGE. Further reports should be accumulated to determine how often patients with FD have VSA and how frequently VF develops in patients with FD and VSA.
Conclusion
Vasospastic angina pectoris should be considered in patients with FD without LGE who experience unexplained VF.
Lead author biography

Kenji Kodama, MD, is Chief physician of Cardiovascular Medicine at Nagahama Red Cross Hospital. He achieved the bachelor degree in medicine from Shiga University of Medical Science. His research focuses on percutaneous coronary and peripheral arterial interventions.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Supplementary Material
Acknowledgements
The authors would like to thank Enago (www.enago.jp) for the English language review.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
References
- 1. Weidemann F, Ertl G, Wanner C, Krämer J.. The Fabry cardiomyopathy-diagnostic approach and current treatment. Curr Pharm Des 2014;21:473–478. [DOI] [PubMed] [Google Scholar]
- 2. Akhtar MM, Elliot PM.. Anderson-Fabry disease in heart failure. Biophys Rev 2018;10:1107–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nakagawa N, Maruyama H, Ishihara T, Seino U, Kawabe J-I, Takahashi F, Kobayashi M, Yamauchi A, Sasaki Y, Sakamoto N, Ota H, Tanabe Y, Takeuchi T, Takenaka T, Kikuchi K, Hasebe N.. Clinical and genetic investigation of a Japanese family with cardiac Fabry disease. Identification of a novel α-galactosidase A missense mutation (G195V). Int Heart J 2011;52:308–311. [DOI] [PubMed] [Google Scholar]
- 4. Ogawa T, Kawai M, Matsui T, Seo A, Aizawa O, Hongo K, Shibata T, Yoshida S, Okamura T, Nishikawa T, Kasajima T.. Vasospastic angina in a patient with Fabry’s disease who showed normal coronary angiographic findings. Jpn Circ J 1996;60:315–318. [DOI] [PubMed] [Google Scholar]
- 5. Krämer J, Niemann M, Störk S, Frantz S, Beer M, Ertl G, Wanner C, Weidemann F.. Relation of burden of myocardial fibrosis to malignant ventricular arrhythmias and outcomes in Fabry disease. Am J Cardiol 2014;114:895–900. [DOI] [PubMed] [Google Scholar]
- 6. Weidemann F, Maier SKG, Störk S, Brunner T, Liu D, Hu K, Seydelmann N, Schneider A, Becher J, Canan-Kühl S, Blaschke D, Bijnens B, Ertl G, Wanner C, Nordbeck P.. Usefulness of an implantable loop recorder to detect clinically relevant arrhythmia in patients with advances Fabry cardiomyopathy. Am J Cardiol 2016;118:264–274. [DOI] [PubMed] [Google Scholar]
- 7. Takenaka T, Teraguchi H, Yoshida A, Taguchi S, Ninomiya K, Umekita Y, Yoshida H, Horinouchi M, Tabata K, Yonezawa S, Yoshimitsu M, Higuchi K, Nakao S, Anan R, Minagoe S, Tei C.. Terminal stage cardiac findings in patients with cardiac Fabry disease: an electrocardiographic, echocardiographic, and autopsy study. J Cardiol 2008;51:50–59. [DOI] [PubMed] [Google Scholar]
- 8. Weidemann F, Niemann M, Störk S, Breunig F, Beer M, Sommer C, Herrmann S, Ertl G, Wanner C.. Long-term outcome of enzyme-replacement therapy in advanced Fabry disease: evidence for disease progression towards serious complications. J Intern Med 2013;274:331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nakamori S, Dohi K, Ishida M, Goto Y, Imanaka-Yoshida K, Omori T, Goto I, Kumagai N, Fujimoto N, Ichikawa Y, Kitagawa K, Yamada N, Sakuma H, Ito M.. Native T1 mapping and extracellular volume mapping for the assessment of diffuse myocardial fibrosis in dilated cardiomyopathy. JACC Cardiovasc Imaging 2018;11:48–59. [DOI] [PubMed] [Google Scholar]
- 10. Chimenti C, Morgante E, Tanzilli G, Mangieri E, Critelli G, Gaudio C, Russo MA, Frustaci A.. Anigina in Fabry disease reflects coronary small vessel disease. Circ Heart Fail 2008;1:161–169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.