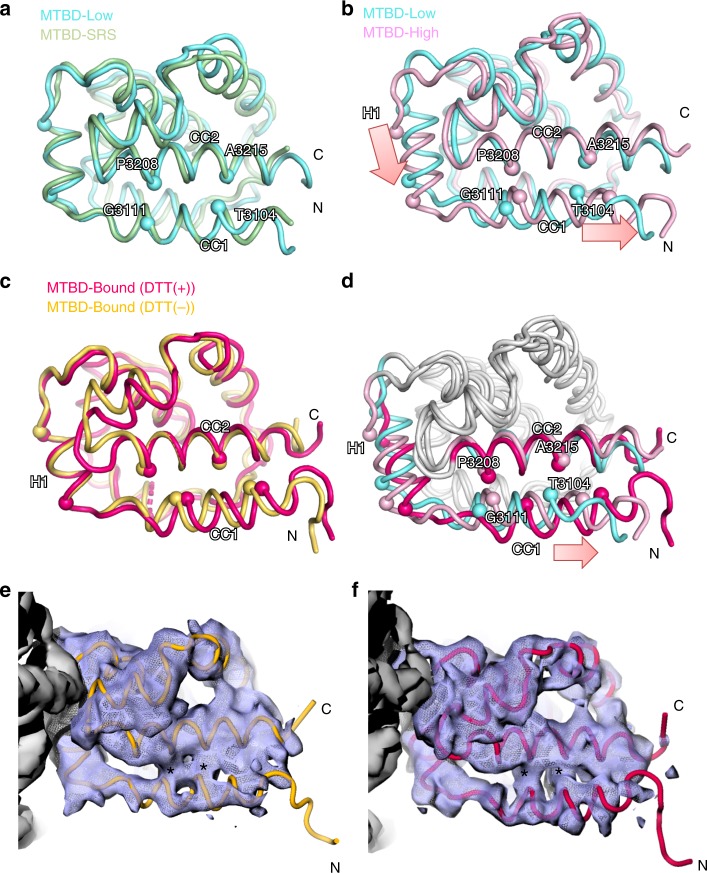
Fig. 2. Structure comparison of MTBD-High, MTBD-Low and MTBD in complex with MT.
a Superposition of MTBD-Low (cyan) and mouse MTBD SRS chimera (PDB code 3EER, light green) viewed from the side. b Superposition of MTBD-High (pink) and MTBD-Low (cyan). c Superposition of cryo-EM model of MTBD in complex with MTs in the DTT(−) (orange) and DTT(+) (magenta) conditions. d The superposition of MTBD-Low (cyan), MTBD-High (pink), and MTBD-Bound (magenta). Only the CC1, H1, and CC2 moieties are colored. The Cα positions of CC1 and CC2 residues that signify the coiled-coil registry are shown by spheres. The notable conformational changes of H1 and CC1 are indicated by pink arrows. e, f Superposition of the cryo-EM map filtered to 4 Å and the model of MTBD in complex with MTs in (e) DTT(−) (yellow) and in (f) DTT(+) (magenta) conditions. Asterisks (*) are placed on the regions corresponding to the hydrophobic interactions between CC1 and CC2 in both maps.