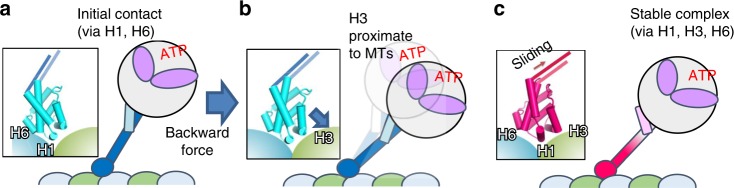
Fig. 7. A model for the directional bias for the applied forces and movement direction of dynein.
a MTBD of dynein makes an initial attachment via a weak-binding interface (H1 and H6). b Backward force or diffusive search in the forward direction tilts MTBD, rendering H3 proximate to the binding site of β-tubulin (indicated by an arrow). c MT binding by MTBD via a strong-binding interface (H1, H3, and H6) induces the sliding of CC1, which would transmit the signal toward the ATPase domain to facilitate ATP hydrolysis and the release of Pi.