Abstract
With improvements in apheresis collection, platelet additive solution (PAS) is steadily replacing plasma as the storage medium in single donor platelets (SDP). Concentrating platelets in SDP with one-third of plasma and two-thirds of PAS is referred as Concentrated-SDP (C-SDP). We studied the influence of donor hematocrit (Hct) in C-SDP procedures. A retrospective study, consisting of 124 and 95 plateletpheresis donors in MCS+ and Trima respectively. We compared two apheresis equipments MCS+ and Trima with regard to donor hematocrit on procedural parameters such as collection efficiency (CE), collection rate (CR), yield per hour (Y/H), yield per litre (Y/L) and percentage blood volume processed (%BV) during C-SDP procedures. Donors were categorized into two groups with Group A (Hct ≤ 46%) and Group B (Hct > 46%) based on mean baseline Hct of the study population. Among the 219 procedures, the overall CE was significantly higher for Trima over MCS+ equipment (77 vs 56, P < 0.001). However, there was no difference in procedural outcomes like CE, Y/L, Y/H, CR with MCS+ or Trima equipment between groups. %BV processed had a negative correlation with hematocrit in MCS+ (r = − 0.305, P = 0.001) and no difference was observed with Trima equipment. Donor Hct influences C-SDP collection only in processed blood volume with MCS+ equipment. Trima had statistically better performance over MCS+ equipments in all procedural parameters during C-SDP procedures. The data will guide apheresis centre to choose equipments based on donor characteristics.
Keywords: Apheresis, SDP, Collection efficiency, Plateletpheresis, Hematocrit
Introduction
Single donor platelets (SDP) transfusion remains one of the most important supportive therapies for thrombocytopenic patients with bone marrow failure [1]. Platelet (PLT) recovery in the patient is influenced by the transfused dose as well as quality of the SDP. The new generation cell separators have made it possible to obtain high quality platelets with minimum donor manipulation [2].
Platelets are usually suspended in plasma, which has been found to be adversely influence the quality of platelets. Platelet additive solution (PAS) are becoming increasingly popular for storage of platelets and steadily replacing plasma as the storage medium [3]. For suspending PAS during apheresis, the platelets were concentrated with one-third (35%) of plasma and two-third (65%) with PAS and the product commonly referred as Concentrated SDP (C-SDP) [4].
PAS are electrolyte solutions used to modulate the quality of the platelets by adding specific ingredients like acetate, potassium, magnesium [3]. SDP stored in PAS result in about half the number of allergic transfusion reactions as compared with platelets suspended in plasma [3, 4]. Recent data suggest that recovery and survival after transfusion as well as corrected count increments by transfusing platelets suspended in PAS may be even better than platelets stored in plasma [3].
Literature review has shown, pre donation platelet count has a direct relationship with platelet yield [1, 2, 5–7]. However, influence of baseline hemoglobin (Hb) or hematocrit (Hct) concentration with platelet yield had no uniform results in standard SDP collection [6–10]. There are limited studies related to donor characteristic that influences the procedural parameters during C-SDP collection. Hence, we aimed to study the influence of donor hematocrit during C-SDP collection procedures between two apheresis devices and this would allow better candidates for the apheresis device to obtain higher platelet yield.
Materials and Methods
In this retrospective study, 124 and 95 procedure details of C-SDP obtained by MCS+ (Haemonetics, Braintree, MA, USA) and Trima Accel (Terumo BCT, Lakewood, CO, USA) systems respectively between March 2017 and September 2018 were included.
All donors met national guidelines for apheresis donation and due consent obtained after details of plateletpheresis were explained to each donor. Criteria for eligibility were as follows: (i) age 18–60 years, (ii) pre-apheresis PLT count ≥ 150 × 109/L with calculated post-apheresis PLT count > 100 × 109/L, (iii) Hb level ≥ 12.5 g/dL, (iv) donor body weight ≥ 60 kg. Donors were sequentially assigned to the MCS+ or Trima device. Senior apheresis technicians under supervision of Medical Officers performed all procedures. Prominent antecubital veins were identified and used for the venipuncture in all donors. None of the donors received prophylactic calcium during the study.
With MCS+ cell separator the universal platelet protocol (UPP) introduces Super Surge® technology, in which during every cycle except the last cycle, platelets collected in the Air-Intermediate platelet bag. During the last cycle, collected platelets are recirculated back into the bowl and creates larger platelet layer than in previous cycles. The system then collects the platelet layer in a small volume of plasma to create the concentrated platelet product. Concentrated platelets in plasma further suspended in the platelet additive solution as mentioned by the manufacturer [5, 11].
Altering the product volume during procedure can impact final product concentration and lowering system’s predicted product volume may increase the final PLT concentration and platelet clumping. With Trima, the procedure was modified by the centre to collect desired platelet yield suspended with one-third of product volume using donor’s plasma at a concentration not exceeding 4.5 × 106/µL during the procedure. At the end of collection, manually adding two-third of product volume with PAS using sterile connecting device with the final PLT concentration in the product bag not exceeding 1.0 to 2.1 × 106/µL as per manufacturers recommendations [12].
Target for platelet yield was uniformly set at 6.0 × 1011 for Trima and for MCS+ the yield was set according to procedure duration with maximum of 120 min.
Complete blood counts (CBC) were determined using an LH750 automated blood cell counter (Beckman Coulter) on donor peripheral blood sample drawn before plateletpheresis procedure and from product bag 1 h post collection. The platelet yield in the product bag was calculated by multiplying the PLT count from cell counter and bag volume. Post procedure platelet count was determined from the apheresis equipment. The post procedure platelet count was validated with the cell counter value before the start of study as described by Bibekov et al. [13]. The following details were recorded from apheresis record: procedure duration, the processed blood volume to reach the desired PLT yield and the acid citrate dextrose-A (ACD-A) volume used.
For studying the influence of donor hematocrit on procedural parameters, donors were categorized into two groups viz Group A (Hct ≤ 46%) and Group B (Hct > 46%) based on their mean baseline hematocrit of the study populationas described by Landzo et al. [14]. Procedural parameters such as collection efficiency (CE), collection rate (CR), yield per liter(Y/L), yield per hour (Y/H), %Blood Volume processed were calculated for both groups and compared between two apheresis devices [15, 16].
Double data entry was done in EpiData entry (V3.1) and compared for discrepancies. A duplicate version of the finalized database was used for statistical analysis using SPSS software version 22 (SPSS Inc, III Chicago, USA). Continuous variables such as PLT yield, CE were presented as mean with standard deviation [mean(SD)]. The independent sample ‘t’ test was used to determine differences between the two groups for all processing and component values. Pearson correlation coefficient was performed to evaluate the correlation such as Hct and PLT yield. A two-tailed p valueof less than 0.05 was considered significant.
Results
Donor characteristics of both groups were comparable in terms of weight, height, blood volume, hematocrit (P > 0.05) and PLT precount was significantly higher with MCS+ equipment (P = 0.019) (Table 1).
Table 1.
Comparison of baseline donor characteristics, product and procedural details between MCS+ and Trima equipments in C-SDP procedures [Mean (SD)]
| Variables | MCS+ (Mean ± SD) | Trima (Mean ± SD) | P Value |
|---|---|---|---|
| Number of donors (n) | 124 | 95 | |
| Weight (Kg) | 76.5 (10.6) | 76.9 (10.2) | 0.733 |
| Height (cm) | 171.9 (6.2) | 170.7 (5.5) | 0.136 |
| Donor blood volume (mL) | 4920.1 (498.9) | 4910.6 (432.1) | 0.883 |
| Hematocrit (%) | 46.7 (2.8) | 46.3 (2.8) | 0.234 |
| Preplatelet count (*103/µL) | 301.7 (42.1) | 288.4 (39.8) | 0.019 |
| Procedure duration (min) | 102.7 (16.4) | 76.3 (16.2) | < 0.001 |
| Post platelet count (× 103/µL) | 206.6 (37.5) | 189.6 (38.8) | 0.001 |
| Anticoagulant used (ml) | 463.6 (60.0) | 397.7 (55.3) | < 0.001 |
| Blood volume processed (mL) | 3546.2 (616.4) | 3285.7 (665.7) | 0.03 |
| Platelet yield (× 1011) | 5.1 (0.8) | 6.0 (0.4) | < 0.001 |
| Product volume (mL) | 393.9 (28.3) | 389.0 (26.8) | 0.190 |
| CE (%) | 56 (6) | 77 (3) | < 0.001 |
| Y/L (× 109/L) | 142.6 (26.6) | 182.7 (26.9) | < 0.001 |
| Y/H (× 1011/min) | 3.0 (0.5) | 5.0 (1.2) | < 0.001 |
| CR (× 1011/min) | 0.05 (0.008) | 0.08 (0.019) | < 0.001 |
| BV processed (%) | 72.9 (16.8) | 67.4 (15.0) | 0.010 |
There were significant differences between MCS+ and Trima concerning procedure duration, post platelet count, anticoagulant volume, donor blood volume processed and platelet yield (Table 1). However, there was no differences in final product volume between the two systems (P = 0.190).
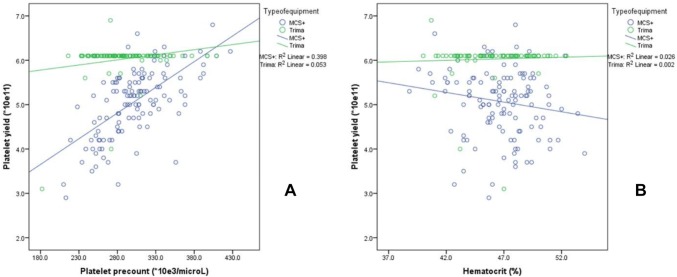
Platelet precount had a positive correlation with platelet yield in MCS+ (r = 0.631, P < 0.001) and Trima (r = 0.229, P < 0.05). However, hematocrit had no such correlation with platelet yield in both the equipments (MCS+ (r = − 0.162, P = 0.074) and Trima (r = 0.050, P = 0.631)) (Fig. 1).
Fig. 1.
Scatterplot between platelet precount (a) and hematocrit (b) with platelet yield between MCS+ and Trima Equipments
The overall CE was significantly higher for Trima over MCS+ equipment (77 vs 56, P < 0.001). There was significant difference in Y/H, CR due to shorter procedure duration with Trima equipment (Table 1). The percentage blood volume processed was significantly higher with MCS+ (73.3 vs 67.4, P = 0.008), while Y/L was low with MCS+ than Trima (142.6 vs 182.7, P < 0.001) (Table 3).
Table 3.
Influence of donor HCT on procedural parameters between MCS+ and Trima equipments in C-SDP collection [Mean (SD)]
| Parameters | Group A | Group B | ||||
|---|---|---|---|---|---|---|
| MCS+ | Trima | P value | MCS+ | Trima | P value | |
| PLT yield (× 1011) | 5.1 (0.8) | 6.0 (0.3) | < 0.001 | 5.0 (0.7) | 6.0 (0.4) | < 0.001 |
| CE (%) | 56 (9) | 76 (4) | < 0.001 | 56 (3) | 78 (2) | < 0.001 |
| Y/L (× 109/L) | 138 (29) | 184 (27) | < 0.001 | 146 (24) | 181 (27) | < 0.001 |
| Y/H (× 1011/min) | 3.0 (0.5) | 5.0 (1.3) | < 0.001 | 2.9 (0.6) | 4.8 (1.1) | < 0.001 |
| CR (× 1011/min) | 0.05 (0.07) | 0.08 (0.02) | < 0.001 | 0.04 (0.09) | 0.08 (0.02) | < 0.001 |
| BV processed (%) | 77 (19) | 68 (12) | 0.02 | 70 (15) | 67 (18) | 0.296 |
With respect to group A and B, comparison within the equipments, there was no difference in procedural outcomes like CE, Y/L, Y/H, CR with MCS+ or Trima equipment. The percentage blood volume processed was significantly lower with Group B donors undergoing apheresis with MCS+ equipment (P = 0.010). However, no such difference in percentage blood volume processed was observed in donors of both groupswith Trima equipment (Table 2) (Fig. 2).
Table 2.
Procedural parameters between MCS+ and Trima equipment with two groups of hematocrit levels in C-SDP procedures [Mean (SD)]
| Parameters | MCS+ | Trima | ||||
|---|---|---|---|---|---|---|
| Group A | Group B | P value | Group A | Group B | P value | |
| PLT yield (× 1011) | 5.1 (0.8) | 5.0 (0.7) | 0.271 | 6.0 (0.3) | 6.0 (0.4) | 0.701 |
| CE (%) | 56 (9) | 56 (3) | 0.943 | 76 (4) | 78 (2) | 0.090 |
| Y/L (× 109/L) | 138 (29) | 146 (24) | 0.090 | 184 (27) | 181 (27) | 0.487 |
| Y/H (× 1011/min) | 3.0 (0.5) | 2.9 (0.6) | 0.602 | 5.0 (1.3) | 4.8 (1.1) | 0.524 |
| CR (× 1011/min) | 0.05 (0.07) | 0.04 (0.09) | 0.602 | 0.08 (0.02) | 0.08 (0.02) | 0.524 |
| BV processed (%) | 77 (19) | 70 (15) | 0.016 | 68 (12) | 67 (18) | 0.740 |
Fig. 2.
Scatterplot between hematocrit and %Blood Volume processed in MCS+ (a) and Trima (b) equipment
Analysis between the equipments in the group A and B showed, Trima had statistically better performance over MCS+ equipments in all procedural parameters (P < 0.001); except blood volume processed in Group B where no significance was observed (Table 3).
Discussion
Advances in automated cell separators had improved the quality of platelet product collected by apheresis. The newer generation PAS certainly shows an in vitro quality of platelets better than when stored in plasma. Double-dose plateletpheresis can help in providing sufficient blood supply under a situation of limited human resources and diminishing donor population [17]. During the study period, automated PAS collection kit was available for MCS+ and was not available for Trima in the country and hence, a customized protocol for preparation of the C-SDP was adopted in Trima.
The mean (SD) donor hematocrit for the entire study population was 46.5 (2.8). We categorized the donors into Group A (Hct ≤ 46) and Group B (Hct > 46) to test whether donor hematocrit has any effect on procedural parameters during C-SDP preparations using UPP protocol of MCS+ and customized protocol of Trima.
Among the procedural parameters, %BV processed seems to be influenced by the donor hematocrit in MCS+ but not with Trima equipment. However, Yin et al. [18] observed CR correlated well with donor hematocrit in MCS+ equipment. Enein et al. [6] observed donor PLT precount and Hb concentration influenced PLTyield: a higher PLT precount corresponded to higher yields, while Hb showed an inverse relationship. However, in the present study, relationship was observed only between PLT precount and PLT yield in both MCS+ and Trima (Fig. 1).
CE was used to evaluate the apheresis system by comparing the number of platelets collected versus the number that pass through the instrument [5]. Different CE were reported in literature for various equipments [5, 19]. In present study, the CE of MCS+ for plateletpheresis using UPP protocol was comparable with Salvadori et al. (56 vs 58). Similarly the CE for Trima was 77% for customized protocol for C-SDP which was similar for standard SDP as reported in literature 60 to 76 [5, 20]. The mean CE was 20% higher with Trima than that of MCS+.
While CE depends upon on donor characteristics and blood volume processed, CR is determined by processing time and platelet yield. The CR provides a better guide to compare apheresis equipments for collecting platelets across studies as it not influenced by the blood volume processed [5, 19, 21]. CR with Trima equipment was higher than MCS+ for C-SDP procedures in the present study (Table 1). Similarly Yin et al. [18] also observed high CR with Trima over Amicus and MCS+ during standard SDP collections.
The procedure duration and its dependant variables such as Y/H and Y/L processed was better with Trima and the results were comparable with study by Bueno et al. (Table 1). This was because Trima worked on continuous flow centrifugation for platelet collection unlike MCS+.
Higher BV processed are usually accompanied by progressive decrease in ionized calcium level as citrate volume increases [22]. Percentage blood volume processed was relatively higher with MCS+ (72.9% vs 67.4%). There was no significant difference between Group A and Group B for procedural parameters in Trima (Table 2). Donor hematocrit had an inverse relationship with %BV processed in MCS+ (r = − 0.305, P = 0.001), low hematocrit was associated with higher plasma volume processed [1, 6] (Fig. 2).
The procedural parameters such as CE, CR, Y/H, Y/L and %BV processed were better with Trima over MCS+ equipment. CE observed between the apheresis may not be directly comparable as different targets were used based on donor platelet count and estimated procedure duration. Similar studies can be conducted for comparing the CE, using same donor who can donate with both the systems at different times.
Conclusion
We conclude that the donor with low hematocrit had high blood volume processed with MCS+. Trima equipment had better procedural parameters over MCS+ during C-SDP collection. The present study will assist blood centre in selecting apheresis equipment based on donor hematocrit for C-SDP procedures.
Acknowledgements
Authors acknowledge the staff of the blood bank and Mr. Riyas for statistical analysis.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sector.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no competing interests.
Ethical Approval
Being retrospective study, Institutional Review Board (IRB) approved the present study without ethical approval through Ref. No: 1616/1RB-SRC/13/MCC/8-12-2018/2 dated: 20th December 2018.
Informed Consent
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Guerrero-Rivera S, Gutiérrez-Espíndola G, Talavera JO, Meillón-García LA, Pedraza-Echevarría M, Pizzuto-Chávez J. Hemoglobin and platelet count effect on platelet yields in plateletpheresis. Arch Med Res. 2003;34:120–123. doi: 10.1016/S0188-4409(02)00453-8. [DOI] [PubMed] [Google Scholar]
- 2.Das SS, Chaudhary RK, Shukla JS. Factors influencing yield of plateletpheresis using intermittent flow cell separator. Clin Lab Haematol. 2005;27:316–319. doi: 10.1111/j.1365-2257.2005.00714.x. [DOI] [PubMed] [Google Scholar]
- 3.van der Meer PF. PAS or plasma for storage of platelets? A concise review. Transfus Med. 2016;26:339–342. doi: 10.1111/tme.12325. [DOI] [PubMed] [Google Scholar]
- 4.Weisberg SP, Shaz BH, Tumer G, Silliman CC, Kelher MR, Cohn CS. PAS-C platelets contain less plasma protein, lower anti-A and anti-B titers, and decreased HLA antibody specificities compared to plasma platelets. Transfusion. 2018;58:891–895. doi: 10.1111/trf.14523. [DOI] [PubMed] [Google Scholar]
- 5.Salvadori U, Minelli C, Graziotin B, Gentilini I. Single-donor platelet apheresis: observational comparison of the new haemonetics universal platelet protocol with the previous concentrated single donor platelet protocol. Blood Transfus. 2014;12:220–225. doi: 10.2450/2013.0119-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Enein AA, Hussein EA, El SS, Hallouda M. Factors affecting platelet yield and their impact on the platelet increment of patients receiving single donor PLT transfusion. J Clin Apher. 2007;22:5–9. doi: 10.1002/jca.20116. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhary R, Das SS, Khetan D, Sinha P. Effect of donor variables on yield in single donor plateletpheresis by continuous flow cell separator. Transfus Apher Sci. 2006;34:157–161. doi: 10.1016/j.transci.2005.09.040. [DOI] [PubMed] [Google Scholar]
- 8.Goodnough LT, Ali S, Despotis G, Dynis M, DiPersio JF. Economic impact of donor platelet count and platelet yield in apheresis products: relevance for emerging issues in platelet transfusion therapy. Vox Sang. 2003;76:43–49. doi: 10.1046/j.1423-0410.1999.7610043.x. [DOI] [PubMed] [Google Scholar]
- 9.Patel J, Nishal A, Pandya A, Patel P, Wadhwani S. Factors influencing yield of platelet aphaeresis using continuous flow cell separator. Int J Med Sci Public Health. 2013;2:309. doi: 10.5455/ijmsph.2013.2.323-326. [DOI] [Google Scholar]
- 10.Ogata H, Nagashima K, Iinuma N, Hosogaya S, Akabane T. Factors influencing yield of plateletpheresis by discontinuous centrifugation. Transfusion. 1981;21:19–22. doi: 10.1046/j.1537-2995.1981.21682085763.x. [DOI] [PubMed] [Google Scholar]
- 11.Corporation H. Haemonetics®. haemonetics MCS+ universal platelet protocols user manual. P/N 118995-IE, Manual Revision: AA Signy-Centre. Switzerland: Haemonetics International; 2016. [Google Scholar]
- 12.Caridian BCT. CaridianBCT platelet storage recommendations. Lakewood, CO, USA: CaridianBCT, Inc; 2010. pp. 5–6. [Google Scholar]
- 13.Bibekov Z, Burkitbaev Z, Skorikova S, Kenzhin A, Magzumova R. The safe and effective plateletpheresis. J Nurs Care. 2017;06:2015–2018. [Google Scholar]
- 14.Landzo E, Sofo-Hafizovic A, Cetkovic-Basic V. Initial values of donor hematocrit and efficiency of plateletpheresis. Acta Inform Medica. 2013;21:116–119. doi: 10.5455/aim.2013.21.116-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bueno JL, García F, Castro E, Barea L, González R. A randomized crossover trial comparing three plateletpheresis machines. Transfusion. 2005;45:1373–1381. doi: 10.1111/j.1537-2995.2005.00215.x. [DOI] [PubMed] [Google Scholar]
- 16.Keklik M, Keklik E, Kalan U, Ozer O, Arik F, Sarikoc M. Comparison of plateletpheresis on the haemonetics and Trima Accel cell separators. Ther Apher Dial. 2018;22:87–90. doi: 10.1111/1744-9987.12607. [DOI] [PubMed] [Google Scholar]
- 17.Picker SM, Radojska SM, Gathof BS. Prospective comparison of high-dose plateletpheresis with the latest apheresis systems on the same donors. Transfusion. 2006;46:1601–1608. doi: 10.1111/j.1537-2995.2006.00928.x. [DOI] [PubMed] [Google Scholar]
- 18.Yin G, Xu J, Shen Z, Wang Y, Zhu F, Lv H. The relationship of platelet yield, donor’s characteristic and apheresis instruments in China. Transfus Apher Sci. 2013;49:608–612. doi: 10.1016/j.transci.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 19.Picker SM, Radojska SM, Gathof BS. A prospective crossover trial comparing performance and in vitro platelet quality of three new apheresis devices with current equipment. Transfus Med Hemotherapy. 2006;33:520–527. doi: 10.1159/000095749. [DOI] [Google Scholar]
- 20.Keklik M, Korkmaz S, Kalan U, Sarikoc M, Keklik E. Effectiveness of the Trima Accel cell separator in the double dose plateletpheresis. Transfus Apher Sci. 2016;55:240–242. doi: 10.1016/j.transci.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 21.Burgstaler EA, Winters JL, Pineda AA. Paired comparison of Gambro Trima Accel versus Baxter Amicus. Transfusion. 2004;44:1612–1620. doi: 10.1111/j.0041-1132.2004.04129.x. [DOI] [PubMed] [Google Scholar]
- 22.Bolan CD, Greer SE, Cecco SA, Oblitas JM, Rehak NN, Leitman SF. Comprehensive analysis of citrate effects during plateletpheresis in normal donors. Transfusion. 2001;41:1165–1171. doi: 10.1046/j.1537-2995.2001.41091165.x. [DOI] [PubMed] [Google Scholar]