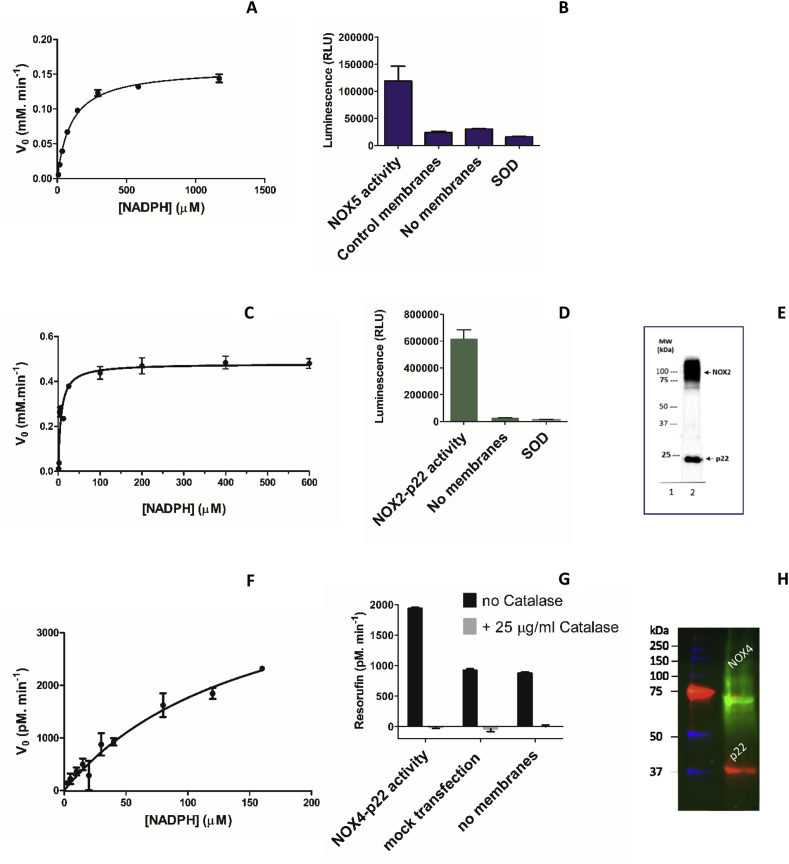
Fig. 2.
Characterization of NOXs. (A) Michaelis-Menten curve of NOX5 membranes using the cytochrome c reduction assay. (B) Superoxide generation by bacterial NOX5 using the MCLA assay. (C) Michaelis-Menten curve of human NOX2-p22 membranes using the cytochrome c reduction assay. (D) Superoxide generation by NOX2 using the MCLA assay. (E) Western-blot analysis of PLB-985 cell membranes by immunoblotting with NOX2 (54.1) and p22 monoclonal (44.1 antibodies). The arrows indicate the highly glycosylated 91 kDa NOX2, and the non-glycosylated 22 kDa p22 (lane 1: molecular weight marker; lane 2: NOX2-p22 containing membranes after separation on 12% polyacrylamide gel electrophoresis). (F) Michaelis-Menten curve of human NOX4-p22 HEK293 membranes using the Amplex Red-peroxidase assay. (G) Hydrogen peroxide generation by NOX4 and corresponding controls using the Amplex Red-peroxidase coupled assay. Full NADPH saturation could not be achieved because of assay interference. (H) In-gel fluorescence analysis of HEK cells heterologously expressing eGFP-NOX4 (predicted 96 kDa) and DsRed-p22 (predicted 50 kDa) expressing membranes. As often observed for membrane proteins, both fusion proteins display anomalous migration in a SDS PAGE due their hydrophobicity. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)