Abstract
The apicomplexan parasite Toxoplasma gondii (T. gondii) causes toxoplasmosis in humans. Pyrimethamine and sulfadiazine that are the drugs of choice to treat the disease, produce severe side effects as well as failure treatments because of drug resistance; thus, novel anti-Toxoplasma compounds are needed and natural compounds can be a good source to obtain them, as medicinal plants have been used to control other apicomplexan parasites. Pleopeltis crassinervata (P. crassinervata) is a fern used in some rural areas of Mexico to treat among other malaises, mouth ulcers, gastrointestinal problems and parasites. Therefore, the efficacy of extracts and fractions obtained from P. crassinervata fronds was evaluated on the viability of T. gondii RH strain tachyzoites by the Stytox green method. RH is the prototypical type 1 Toxoplasma strain, isolated for the first time from the brain of a patient boy named R. H. Its phytochemical profile, MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay, Hep-2 cytotoxicity and antioxidant activity by ABTS (2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) and DPPH (2,2-diphenyl-1-picrylhydrazyl) methods, were also assessed. Hexane fraction exhibited the highest anti-Toxoplasma activity with an IC50 of 16.90 µg/mL. This fraction did not show antioxidant activity and contained at least 2 terpenoid type compounds with retention factor (Rf) of 0.75 and 0.86. The fraction was not toxic to the host cells in doses up to 50 µg/mL. P. crassinervata frond hexane fraction seems to be a good candidate to obtain possible anti-Toxoplasma compounds. This study is the first to report the biological, antioxidant and cytotoxic activity of P. crassinervata fern.
Keywords: Toxoplasma gondii, Natural compounds, Pleopeltis crassinervata, Hexane fraction, Terpenoids
1. Introduction
Toxoplasmosis, a neglected disease caused by the ubiquitous apicomplexan T. gondii, can go clinically unnoticed in immunocompetent hosts or cause serious and fatal complications in immunocompromised patients (Vidal, 2019). T. gondii can produce abortion, encephalitis, and death, and it is considered a major opportunistic pathogen in AIDS patients, so fetus, women of reproductive age and immunocompromised patients are the most affected groups. It is estimated to chronically infect one-third of the world's human population. The parasite can infect other warm-blooded nonhuman animals including terrestrial and aquatic mammals and birds, so it is considered a significant zoonotic and veterinary pathogen. T. gondii can be transmitted by the oral ingestion of food and water contaminated by parasite oocysts that are released in cat feaces, or by the consumption of raw or undercooked meat containing T. gondii tissue cysts (Vidal, 2019).
Up to date, anti-toxoplasma drugs are only useful during the acute phase of the disease which is caused by dissemination of fast-replicating tachyzoites; nonetheless, once tachyzoites undergo stage conversion to slow-growing bradyzoites, tissue cysts are developed (mainly in the brain) and no effective treatment is available against them. Thus, tissue cysts are responsible for chronic infections that can persist for the lifetime of the host, causing subclinical basal neuroinflammation (Lang et al., 2018) or reactivation to an acute phase. As a result of a host immunocompromise, bradyzoites reconvert back to tachyzoites and might cause, among other complications, lethal encephalitis in AIDS patients or congenital toxoplasmosis (Vidal, 2019). Drug resistant and toxicity, the absence of human vaccine, as well as the emergence of atypical T. gondii strains, are some factors that complicate toxoplasmosis control (Montazeri et al., 2018). Pyrimethamine-sulfadiazine is the combination of choice to treat acute toxoplasmosis. Nevertheless, these drugs can cause severe side effects in the host as agranulocytosis, hypersensitivity reactions, leucopenia, neutropenia, skin and hepatic necrosis, Stevens-Johnson syndrome and thrombocytopenia; in addition to this, resistance has been reported for both drugs (Mandelbrot et al., 2018). In silico drug design might seem an appropriate alternative in the search of new compounds against T. gondii (Rivera et al., 2016), however, until now, there has been no release of any new anti-toxoplasma compound into the market. Since the beginning of humankind, plants have been used to treat different diseases, in fact, two of the most widely used antimalarials (chloroquine and artemisinin derivatives) were synthetized from quinine and artemisinin compounds obtained from plants. Therefore, natural compounds could be an important source to investigate novel anti-Toxoplasma molecules as T. gondii shares some biological and structural characteristics with malaria and other apicomplexan. P. crassinervata (Fig. 1) is a fern belonging to the Polypodiaceae family that is used in some rural areas of Mexico (based on empirical knowledge) to treat mouth ulcers (Fernández et al., 2001), gastrointestinal problems and parasites. In recent studies we observed that P. crassienervata methanolic fractions were active against Trichomonas vaginalis (unpublished data). However, until now, P. crassinervata available scientific data refer only to its taxonomic and biological features. Based on the empirical use of P. crassinervata and on its anti-trichomona activity previously obtained, in the present study, the efficacy of P. crassinervata frond fractions, were evaluated in T. gondii RH strain tachyzoites in order to obtain biological and chemical data that will help in the near feature to obtain a lead natural compound to design new anti-toxoplasma drugs. Phytochemical profile as well as in vitro cytotoxicity and antioxidant activity were also assessed.
Fig. 1.
Image of Pleopeltis crassinervata frond showing their sori (yellowish masses).
2. Materials and methods
2.1. Animals
Five-week BALB/c male mice were obtained from the School of Medicine, UNAM vivarium. Animal management was performed according to the Mexican Official Norm NOM-062-ZOO-1999 for the production, care, and use of laboratory animals in accordance to international guidelines and approved by the Ethical and Research committee at School of Medicine, UNAM (project 052/2017).
2.2. Parasites
RH tachyzoites maintained by intraperitoneal (IP) passages in BLAB/c mice were harvested from intraperitoneal (IP) fluid of infected mice on the 4th day of infection and centrifugated twice (for ten minutes) at 260g. Parasites were rinsed with phosphate-buffered saline (PBS) pH 7.4, filtered through 5 μm membranes and maintained in PBS. Purified tachyzoites were used within 4 h after their isolation (Rivera et al., 2016).
2.3. Cell culture
To host T. gondii tachyzoites, human larynx epidermoid carcinoma epithelial cells (HEp-2, ATCC-CCL 23) maintained in minimum essential media (MEM) supplemented with 10% fetal bovine serum (FBS) under 5% CO2 atmosphere at 37 °C, were used (Rivera et al., 2016).
2.4. Plant material and extraction
P. crassinervata was collected during the reproductive period by J Anacleto (September-October) in Chignautla, Puebla, Mexico (19°50′24.49″N and 97°22′30.08″O). A plant specimen was identified and authenticated by Leticia Pacheco (taxonomist) and deposited in the Metropolitan Herbarium Dr. Ramón Riva and Nava Esparza (UAMIZ) with voucher number 84415. Frond was removed and dried in a recycled air stove at 37 °C and chopped in a windmill with particle mesh size not grater that 5 mm. 100 g of dried powder was subjected to extraction by maceration for 4 to 5 weeks with methanol in a solid-liquid-liquid continuous system. The filtered extract was concentrated in a rotary evaporator to remove the solvent and lyophilized. The extract was filtered, concentrated and stored at −80 °C until used. The lyophilized extract was dissolved in methanol in a 1:10 w/v, and an equivalent volume of hexane was added to the solution. Three fractions were obtained (hexane, methanolic and one precipitate), the solvents were removed in a rotary evaporator and the fractions were lyophilized (Rivera et al., 2014).
2.5. Phytochemical evaluation
To determine phytoconstituents, extract and fractions were analyzed. The presence of alkaloids was determined by a turbidity in the mix suspension (10 g sample plus a few drops of HCl). Tannins were detected when 0.01 g of the sample turned to dark blue after dissolved in 0.2 mL of water with FeCl3 0.1%. Samples were mixed with 0.4 mL of acetic anhydride and 0.4 mL of sulfuric acid, turned to a red violet color when steroids were detected. Samples (10 mg) dissolved in 0.4 mL of chloroform and 0.6 mL of sulfuric acid, formed brown rings when terpenoids were found. By mixing the samples in ethanolic FeCl3 flavonoids were detected by a red color. For total phenols, 5 mL of solution from the samples, was mixed with FeCl3 solution. The change in color to dark blue indicated the presence of phenols. Saponins were detected by dissolving 10 mg of the extract in 1 mL of distilled water and few drops of olive oil; the formation of emulsion indicated the presence of saponins (Yadav and Agarwala, 2011). As an antioxidant evaluation was made, quantification of total polyphenols was measured by the Folin-Ciocalteu method. The samples were dissolved in distilled water (4 mg/mL). 250 μL of Folin reagent was added to an aliquot of 50 μL of the sample and after 5 min, 1250 μL of sodium carbonate (20%) and 450 μL of distilled water were added. The reaction mixture was kept protected from light at room temperature for 2 h and subsequently the absorbance of the blue complex formed at 760 nm was recorded. The absorbance value was compared with a standard curve of gallic acid (20–200 μg/mL) and the result was expressed as grams of gallic acid/100 g of dry extract (Tadesse et al., 2015). A mixture of water and the reagents were used as a blank. All determinations were made in triplicate and the mean value was calculated. Hexane fraction was analyzed by thin layer chromatography (TLC) performed on silica gel plates (60 GF254, Merck) and washed out with hexane: ethyl acetate in 7: 3 ratio. The plates were visualized with ultraviolet light (254 and 365 nm). Vanillin: H2SO4 (reagent used for terpenoids detection) was applied to the plates. The plate sprinkled with Vanillin: H2SO4 was heated at 100° C until the spots appeared. Terpenoids identification was made following the method described by Wagner et al. (1984).
2.6. Effect of P. Crassinervata on the viability of extracellular tachyzoites
Parasites viability was determined based on their capability to exclude Sytox Green® stain. Purified tachyzoites (1 × 106) were incubated in suspension with methanolic extract and fractions of P. crassinervata that were first dissolved in dimethyl sulfoxide (DMSO) 0.04% and then diluted in serum-free MEM to final concentrations of 5–100 µg/mL for 1 h at room temperature and then Sytox Green (1:500) was added for 15 min; parasites were counted in a fluorescence microscope (Castro et al., 2018). The experiment was done in triplicate counting 300 tachyzoites in independent events. Concentrations were determined according to the IC50 obtained in previous studies regarding P. crassinervata in vitro activity on Trichomonas spp (Unpublished data) and according to IC50 concentrations obtained regarding the efficacy of natural compounds against T. gondii RH strain tachyzoites (Nasr et al., 2016). Methanolic extract was chosen based on the same fundaments. Untreated tachyzoites maintained in MEM or 0.04% DMSO were used as control groups. Pyrimethamine was used as positive control at 10 µg/mL (van der Ven et al., 1996).
2.7. Effect of P. Crassinervata on uninfected HEp-2 cells and MTT assay
To assess the toxicity on the host cells, HEp-2 cells were grown for 24 h in MEM + 10% FBS and then exposed to 5–500 µg/mL of P. crassinervata hexane fraction for 24 h at 37 °C. Cells were rinsed once with MEM and then stained with Sytox Green. Viability percentage of HEp-2 cells was determined by counting the 300 cells that excluded the stain. The experiment was carried out in triplicate at independent events. Viability cell was also assessed by the 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) test in SH-SY5Y human neuroblastoma cell line (ATCC® CRL-2266™) at higher hexane fraction concentrations (25–750 µg/mL) for 24 h (van Meerloo et al., 2011).
2.8. Antioxidant assay by radical cation (ABTS•+) and Antioxidant assay by DPPH• method
ABTS and DPPH are the most suitable methods for the determination of antioxidant activity of natural compounds. On the ABTS the activity of hydrophilic and lipophilic compounds can be measured, while DPPH can only be dissolved in organic media (Bavoba et al., 2016)
The radical ABTS•+ was obtained from the reaction of an ABTS solution (7 mM) in potassium persulfate (2.45 mM). The reaction was carried out at room temperature and in the dark for 16 h. Once the radical ABTS•+ was formed, it was diluted until obtaining an absorbance value comprised around 0.70 (±0.2). From stock solutions of methanolic extract and its fractions at concentrations of 5 mg/mL in DMSO, decreasing dilutions were prepared at concentrations ranging from 1 to 500 μg/mL (Re et al., 1999). The assay was carried out in a 96 well microplate, in each well 100 μL of ABTS•+ solution and 100 μL of the sample were added, each experiment was carried out in triplicate. Finally, absorbance readings were made at 743 nm in a plate recorded every 3 min for one hour, a mixture was used as blank of 100 μL of ABTS•+ solution and 100 μL of DMSO. The percent inhibition was calculated according to the following equation through reaction kinetics:
Where A = Absorbance of blank and A1 = Absorbance of sample
A solution of the DPPH• + radical at 200 μM was prepared. From stock solutions of methanolic extract and their fractions [5 mg/mL in DMSO], decreasing dilutions were prepared in concentrations of 1 to 500 mg/mL. In a 96-well microplate, 150 μL of DPPH• + Solution and 50 μL of the sample were deposited in each well in triplicate; and, finally, absorbance readings were taken at 520 nm in a plate reader every 3 min for one hour. A mixture of 150 μL of ABTS•+ solution and 50 μL of DMSO was used as blank (Molineux, 2004). Antioxidant capacity was evaluated as percentage of inhibition according to the previous equation.
3. Statistics
Obtained data were analyzed with one-way analysis of variance (ANOVA) and Tukey’s test using GraphPad Prism® software, version 7. All analyzed data had a normal distribution, and statistical significance was set at P < 0.05. Half minimal inhibitory concentration was calculated by nonlinear regression analysis and viability percentages were expressed as mean ± standard error of three replicates.
4. Results
For each 100 g of dry weight, a yield of 48.28% was obtained from the methanolic extract of P. crassinervata frond. Hexane fraction yield was the lowest (1.20%) when compared with the yields of methanolic (31.21%) and precipitate (10.41%) fractions. Yields obtained from each fraction with respect to the methanolic extract are shown in Table 1. The phytochemical analysis showed the presence of different types of organic molecules. In the methanolic extract tannins, flavonoids and alkaloids were observed, and in the total polyphenols quantification it turned to be the sample with the highest quantity of them, with a concentration of 15.14% equivalents of gallic acid. Precipitate fraction was positive to 7 of the 8 phytochemical qualitative tests, showing the presence of terpenoids, tannins, saponins, flavonoids, alkaloids and polyphenols and presented 14.38% of total equivalent polyphenols of gallic acid. The methanolic fraction showed the presence of tannins, flavonoids, alkaloids and 14.06% of total equivalent polyphenols of gallic acid. The hexane fraction was positive for terpenoids, this fraction contains at least two terpenoid type compounds as with the vanillin: H2SO4, two green-blue spots with Rf of 0.75 and 0.86 were observed. Phytoconstituents can be observed in Table 2.
Table 1.
Pleopeltis crassinervata extraction yields.
| Yield g/100 g of methanolic extract | Total yield % | |
|---|---|---|
| Hexane fraction | 1.20 | 2.48 |
| Methanolic fraction | 31.23 | 64.62 |
| Precipitate | 10.41 | 21.56 |
Yields obtained from the fractions of P. crassinervata with respect to methanolic extract, expressed in percentage and in grams per 100 g of methanolic extract.
Table 2.
Phytochemical analysis of Pleopeltis crassinervata.
| Assay | Methanolic extract | Hexane Fraction | Methanolic fraction | Precipitate |
|---|---|---|---|---|
| Steroids | — | + | — | — |
| Terpenoids | — | — | — | + |
| Tannins | + | — | + | + |
| Saponins | — | — | — | + |
| Flavonoids | + | — | + | + |
| Alkaloids (Drangerdof reagent) | + | + | + | + |
| Alkaloids (Mayer reagent) | + | + | + | + |
| Alkaloids (Wagner reagent) | + | — | + | + |
| Quantification of total polyphenols (% equivalent of gallic acid) | 15.14 ± 0.33 | 0 | 14.06 ± 0.57 | 14.38 ± 0.33 |
Results of the qualitative phytochemical tests and quantification of total polyphenols expressed in gallic acid equivalent percentage for the methanolic extract and its fraction. Positive (+), Negative (−).
4.1. Effect of P.crassinervata in T. gondii tachyzoites and cell viability
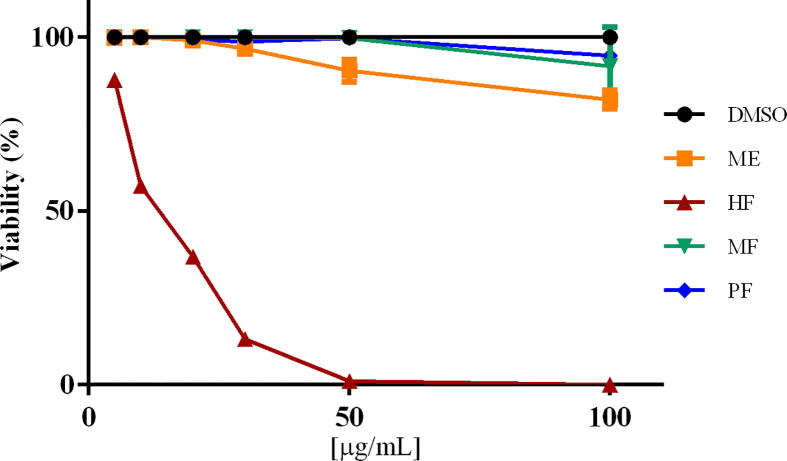
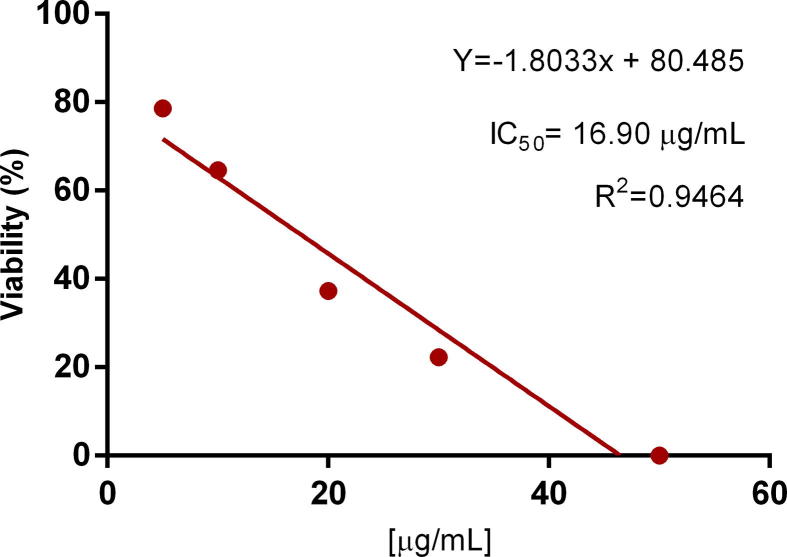
Viability assays were performed with Sytox® green, a nucleic acid stain very useful in cell toxicity as it distinguishes dead from live cells. Dead cells bright in fluorescence green (excitable at 488 nm with an emission profile like FITC) since the stain enters the damaged membrane and binds to DNA. Sytox green has been used in different protocols to assess T. gondii tachyzoites viability (Castro et al., 2018, Pavlou et al., 2018). Methanolic extract and the hexane fraction affected the viability of T. gondii tachyzoites; nevertheless, methanolic extract was efficient at 100 µg/mL affecting only about 20% of the parasites. At 5 μg/mL, only hexane fraction showed a decrease of about 20% in tachyzoites viability when compared to the untreated tachyzoites maintained in MEM or DMSO-MEM (both controls behaved similarly). The greatest toxic effect on tachyzoites viability was observed with hexane fraction at concentrations of 20 to 100 µg/mL (Fig. 2); at the highest concentration all parasites were completely destroyed. The IC50 for the hexane fraction was of 16.90 µg/mL in comparison with the IC50 of pyrimethamine that was of 14.20 µg/mL.
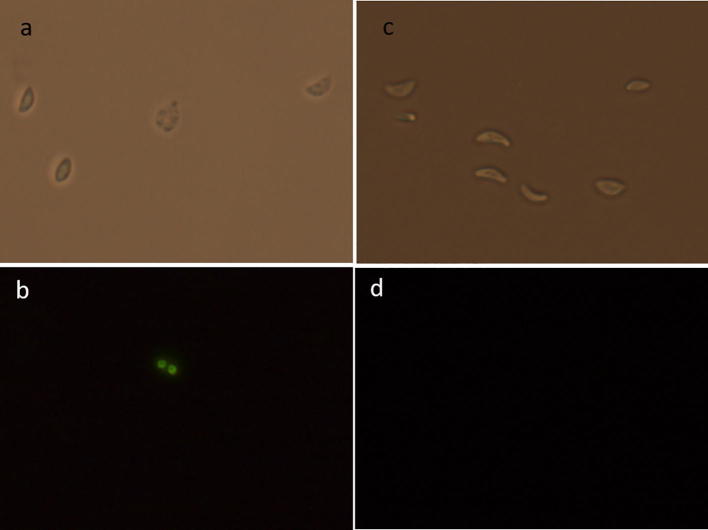
Fig. 2.
Photomicrographs of T. gondii RH strain tachyzoites 40×. (a) Exposed to hexane fraction [25 µg/mL], observed in bright field; (b) observed in fluorescence microscopy; damage parasites are those with green bright nucleus. (c) and (d) control without treatment in bright field and fluorescence microscopy respectively.
Based on the viability results, we decided to evaluate the hexane fraction in Hep-2 cells in order to assess its possible toxicity (Fig. 3). The evaluated concentrations were the same used in the T. gondii viability assay and up to 500 µg/mL. Results showed that the fraction is not toxic to host cells at concentrations up to 50 µg/mL, as with this concentration cell culture presented a confluence greater than 90% and no morphological changes. In some cells treated with 100 µg/mL vacuolization was observed as well as weak cell unions and loss of cell layer integrity, while cell membranes did not present any damage. However, for this reason, MTT cytotoxicity assay (Fig. 4) in Sh-Sy5Y cells was carried out and an IC50 of 240.35 µg/mL was obtained for the hexane fraction. Dead T. gondii tachyzoites stained with Sytox green can be observed in Fig. 4. The effect of P. crassinervata on T. gondii tachyzoites viability (Table 4) as well as IC50 values are shown in Fig. 5, Fig. 6, respectively.
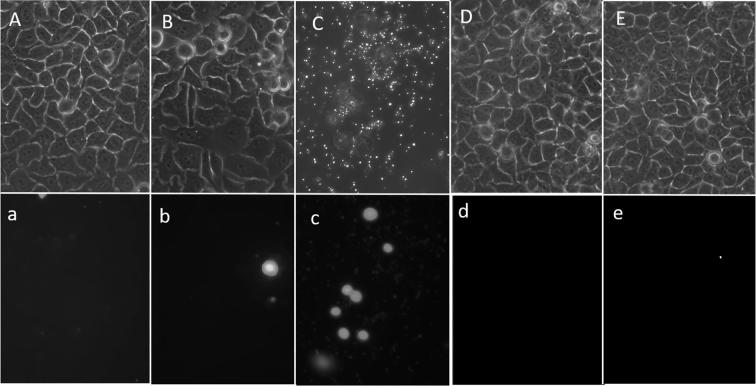
Fig. 3.
Photomicrographs of Hep-2 cell cultures exposed to P. crassinervata hexane fraction at concentrations from 50 to 400 µg/mL, Sytox green® dye was used as developer; from 400 µg/mL cell cultures become destroyed, only cell debris and some suspended cells were observed. Hexane fraction phase contrast images: A: 25 µg/fraction; B: 100 µg/mL, C: 400 µg/mL, D: 0.3% Dymethil sulfoxide control. Boxes marked with lowercase letters showed fluorescence images, damage cells with fluorescence nucleus can be observed in b and c boxes.
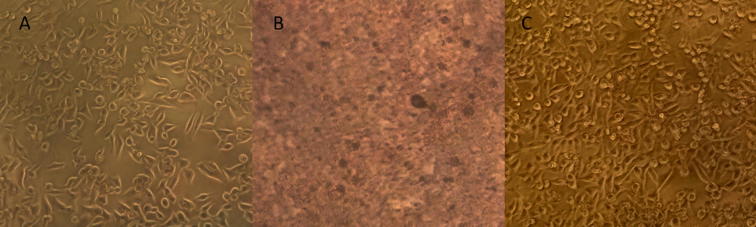
Fig. 4.
Sh-Sy5Y cells exposed to different concentrations (25–1000 µg/mL) of P. crassinervata hexane fraction. (A) hexane fraction 25 µg/mL, (B) hexane fraction 500 µg/mL, (C) Dimethyl sulfoxide control. Cells became unviable from 400 µg/mL.
Table 4.
Efficacy of Pleopeltis crassinervata on Toxoplasma gondii tachyzoites viability.
| Concentration (µg/mL) | DMSO | Methanolic extract | Hexane fraction | Methanolic fraction | Precipitate fraction |
|---|---|---|---|---|---|
| 5 | 100 | 99.66 ± 0.33 | 87.66 ± 2.03* | 100 | 100 |
| 10 | 100 | 100 | 57.11 ± 0.58* | 100 | 100 |
| 20 | 100 | 99.00 ± 0.57 | 36.77 ± 1.44* | 100 | 99.33 ± 0.33 |
| 30 | 100 | 96–66 ± 0.33 | 13.11 ± 0.58* | 100 | 98.66 ± 0.88 |
| 50 | 100 | 90,33 ± 1.85 | 1.08 ± 0.78* | 99.66 ± 0.33 | 99.66 ± 0.33 |
| 100 | 100 | 82.00 ± 1.52* | 0* | 91.66 ± 2.43 | 94.66 ± 0.66 |
Results are the mean ± standard error of three independent studies. P < 0.05.
Statistical significance versus control group
Fig. 5.
Tachyzoites viability percentage after one-hour exposure to P. crassinervata ME: methanolic extract; HE: hexane fraction; MF: methanolic fraction; PF: precipitate fraction. DMSO (Dimethyl sulfoxide).
Fig. 6.
IC50 value was calculated with a linear regression as dose-response curve had a linear behavior.
4.2. Antioxidant activity
IC50 values obtained from the ABTS assay were of 15.41, 82.01, 37.08 and 25.16 μg/mL for the precipitated, hexane fraction, methanolic fraction and methanolic extract respectively, in the first absorbance reading. The percentage inhibition values were plotted as a function of time for each concentration evaluated in the ABTS and DPPH assays, these values are summarized in Table 3. The fraction with the highest antioxidant capacity was the precipitated with an inhibition percentage of 86% in the ABTS assay and 76% in the DPPH assay at a concentration of 35 and 150 μg/mL, respectively. The hexane fraction showed the lowest antioxidant capacity with a percentage maximum inhibition of 34% in the ABTS assay and 56% in the DPPH assay when adding a concentration of 75 μg/mL to the sample. The methanolic fraction was also active but in a lower degree, with a percentage of inhibition of 58% for the ABTS test and 73% for DPPH with a concentration of 50 and 300 μg/mL, respectively. Methanolic extract showed a 76% inhibition when applying 150 μg/mL of the extract in the DPPH assay and 85% in the ABTS assay. The maximum inhibition percentage was at 30 min after of the first absorbance reading for the ABTS assay. In the present study both assays ABTS and DPPH were used as P. crassinervata extract and fractions have different polarities, nonetheless, fern components have higher affinity with ABTS.
Table 3.
Pleopeltis crassinervata antioxidant capacity.
| Fraction | % maximum inhibition | Stabilization time (min) | Minimum concentration for stabilization (µg/mL) | % maximum inhibition at 1 μg/mL | ||||
|---|---|---|---|---|---|---|---|---|
| ABTS | DPPH | ABTS | DPPH | ABTS | DPPH | ABTS | DPPH | |
| Precipitate | 86 ± 1.76 | 76 ± 1.20 | 24 | N/E | 35 | 150 | 20 ± 1.00 | 6 ± 0.88 |
| Hexane | 34 ± 2.33 | 56 ± 2.00 | 24 | N/E | 50 | 75 | 6 ± 0.57 | 6 ± 0.66 |
| Methanolic | 58 ± 1.76 | 73 ± 2.18 | 21 | N/E | 50 | 300 | 7 ± 0.33 | 2 ± 0.57 |
| Methanolic total extract | 85 ± 1.55 | 76 ± 2.30 | 21 | N/E | 50 | 200 | 7 ± 0.66 | 6 ± 0.88 |
Evaluated concentrations: 1–500 μg/mL; N/E = not reached.
5. Discussion
In the present study, methanolic extract was used as a starting point to obtain different fractions from the frond of P. crassinervata, as in previous studies made on T. vaginalis, it was the extract that showed the higher antiparasitic efficacy (unpublished data). RH strain tachyzoites is commonly used to assess new compounds against T. gondii because it produces acute toxoplasmosis (Rivera et al., 2016) and for this reason, it was the strain of choice to evaluate the fractions of P. crassinervata. The highest toxoplasmicidal activity was observed with the hexane fraction which contained terpenes, had no antioxidant activity and presented the lowest extraction yield. Some terpenoids such as artemisinin are found in low concentrations in plants (less than 0.01 to 1.4% of the plant dry weight) (Liu et al., 2006). In China, the biological activity and phytochemical composition of different ferns from the Polypodiaceae family (Drynaria quercifolia, P. adnascens and M. punctatum) have been studied (De la Cruz et al., 2017) and the presence of alkaloids, flavonoids, terpenoids and sterols in these plants were reported; flavonoids and terpenoids were the most widespread metabolites in these ferns (De la Cruz et al., 2017, Soeder, 1985). Not much is known about the phytochemistry profile of P. crassinervata. Nonetheless, it seems that our results agree with those reported by other authors regarding the phytoconstituents of the Polypodiaceae family ferns, as in these studies, the presence of flavonoids, alkaloids, terpenoids, tannins and saponins were reported (De la Cruz et al., 2017). It is worth highlighting that the hexane fraction which presented the highest toxicity to the parasite, was positive to terpenoids, being this result in line with previous reports that showed that terpenoids are one of the most common molecules found in these ferns. Not much is reported about the effect of natural compounds against T. gondii; however, in previous studies, diterpenes, tannins, alkaloid, triterpenes and flavonoids obtained from different plants were found to be active against T. gondii RH strain tachyzoites (Nasr et al., 2016). Maybe, the most interesting reference regarding the activity of terpenoids against apicomplexan parasites, could be the antimalarial activity of the artemisinin sesquiterpene lactone derived from Artemisia annua. Artemisinin is also effective against T. gondii (Ho et al., 2014). Terpenoids have also been the origin of some anticancer compounds as paclitaxel (Weathers and Elkholy, 2006) and their efficacy was also evaluated against Leishmania and Trypanosoma (Shah et al., 2009). In our study, hexane fraction depicted an IC50 of 16.90 µg/mL. IC50 values ranging from 0.03 to 1000 µg/mL have been reported for other plants extracts and fractions tested in vitro against T. gondii tachyzoites. The IC50 of pyrimethamine in this study was of 14.20 µg/mL, in comparison with the IC50 obtained in other assay where the value was of 0.2 mg/L of pyrimethamine (approximately the range of plasma concentrations obtained after a once weekly dose of the drug) (Re et al., 1999). IC50 value for sulfadiazine is as high as 600 mg/L. Based on these reports it seems that anti-Toxoplasma IC50 obtained with the hexane fraction of P. crassinervata, falls in a suitable range, and same could be said about cytotoxicity in Hep2 and Sh-Sy5Y cells as in both cases damage was observed at concentrations higher than 50 µg/mL and up to 240.35 µg/mL in comparison with cytotoxicity of pyrimethamine that is observed at 50 µg/mL or lower doses (Nasr et al., 2016).
This is the first study to report the efficacy of P. crassinervata in a biological model, as until now, published data refer only to its taxonomic and genetic aspects (Smith and Tejero, 2014).
Our results showed that the hexane fraction presented the lowest in vitro antioxidant activity, thus it could be assumed that the damage produced in the parasite is not related with an antioxidant activity. It is now known that 75% of the potentiators of antibacterial compounds are terpenes (Zacchino et al., 2017), and even though the exact mechanism of actions of terpenes remains unknown, it is believed that they can inhibit both oxygen uptake and oxidative phosphorylation processes (Grin et al., 1999). Terpenes mode of action include among other mechanisms, the generation of reactive oxygen species (ROS) and other free radicals after an oxidative stress process. In fact, artemisinin antimalarial effect is recently believed to occur as a result of a prooxidant process derived by the accumulation of ROS and DNA damage in the parasite (Gopalakrishnan and Kumar, 2015). Based on these facts, it could be inferred that the anti-Toxoplasma effect of P. crassinervata hexane fraction could be linked to these mechanisms of action.
In conclusion, this study demonstrated for the first time the efficacy of the hexane fraction of P. crassinervata frond against T. gondii tachyzoites as well as its antioxidant and cytotoxic activity. The toxoplasmicidal effect of the P. crassinervata frond hexane fraction on RH tachyzoites was obtained at concentrations comparable to those used with different anti-Toxoplasma drugs that are used to treat acute toxoplasmosis but with less toxicity to the host cells. Nevertheless, more studies need to be done in order to evaluate this fraction as well as its possible characterized compounds on the complete life cycle of T. gondii and in in vivo models. Finally, these results demonstrated that P. crassinervata metabolites can be considered as possible candidates to obtain lead compounds against T. gondii and that natural compounds can be a good source for the development of new antiparasitic drugs. This study also constitutes the first record of chemical-pharmacological research of Pleopeltis crassinervata.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
None.
Acknowledgements
Authors want to thank Mrs. Josefina Bolado, Head of the Scientific Paper Translation Department, from División de Investigación at Facultad de Medicina, UNAM, for editing the English-language version of this manuscript and Leticia Pacheco for determining the herbarium specimen
Footnotes
Peer review under responsibility of King Saud University.
References
- Bavoba O., Occhipinti A., Capuzzo A., Maffei M.E. Extractions of bilberry (Vaccinium mytrillus) antioxidants using supercritical/subcriticalCO2and ethanol as co-solvent. J. Supercrit. Fluids. 2016;107:358–363. [Google Scholar]
- Castro E.K.N., Hernández C.P., Ramírez F.C.J., González P.S., Gómez de León C.T., Mondragón C.M., Mondragón F.R. Mycophenolic acid induces differentiation of Toxoplasma gondii RH strain tachyzoites into bradyzoites and formation of cyst-like structure in vitro. Parasitol. Res. 2018;117:547–563. doi: 10.1007/s00436-017-5738-x. [DOI] [PubMed] [Google Scholar]
- De la Cruz R.Y., Ang A.M., Doblas G.Z., Librando I.L., Porquis H.C., Batoctoy L.S., Cabresos C.C., Jacalan D.R., Amoroso V.B. Phytochemical screening, antioxidant and anti-inflammatory activities of the three fern (polypodiaceae) species in Bukidnon, Philippines. Bull. Env. Pharmacol. Life. Sci. 2017;6:28–33. [Google Scholar]
- Fernández N.R., Ramos Z.D., Carranza G.E. Notas sobre plantas medicinales del estado de Querétaro, México. Polibotánica. 2001;12:1–40. [Google Scholar]
- Gopalakrishnan A.M., Kumar N. Antimalarial action of artesunate involves DNA damage mediated by reactive oxygen species. Antimicrob. Agents. Chemother. 2015;59:317–325. doi: 10.1128/AAC.03663-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grin S.G., Wyllie J.L., Markham D.N. Leach, The role of structure and molecular properties of terpenoids in determining their antimicrobial activity. Flavour. Fragr. J. 1999;14:322–332. [Google Scholar]
- Ho W.E., Peh H.Y., Chan T.K., Wong W.S. Artemisinins: pharmacological actions beyond anti-malarial. Pharmacol. Ther. 2014;142:126–139. doi: 10.1016/j.pharmthera.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Lang D., Schott B.H., van Ham M.L., Kulikovskaja L., Herrera M.R., Pielot R.F., Klawonn F., Montag D., Jänsch, Gundelfinger E.D., Smalla K.H., Dunay I.R. Chronic Toxoplasma infection is associated with distinct alterations in the synaptic protein composition. J. Neuroinflammation. 2018;15:15–216. doi: 10.1186/s12974-018-1242-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Zhao Y., Wang Y. Artemisinin: current state and perspectives for biotechnological production of an antimalarial drug. Appl. Microbiol. Biotechnol. 2006;72:11–20. doi: 10.1007/s00253-006-0452-0. [DOI] [PubMed] [Google Scholar]
- Mandelbrot L.I., Villena F., Kieffer D.H., Laurichesse N., Winer L., Mesnard A., Berrebi G., Le Bouar G.A., Cordier R., Sitta R. Congenital toxoplasmosis prevention by pyrimethamine-sulfadiazine vs spiramycin. a randomized trial. AJOG. 2018;218:S25. [Google Scholar]
- Molineux P. The Use of stable free radical diphenylpicrilhydrayl (DPPH) for estimating antioxidant activity. Sci. Tech. 2004;121:93–101. [Google Scholar]
- Montazeri M.S., Mehrzadi M., Sharif S., Sarvi A., Tanzifi S., Aghayan A. Drug resistance in Toxoplasma gondii. Front. Microbiol. 2018;9:2587. doi: 10.3389/fmicb.2018.02587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasr I., Ahmed F., Pullishery F., El-Ashram S., Ramaiah V. Toxoplasmosis and anti-Toxoplasma effects of medicinal plant extracts-A mini-review. Asian. Pac. J. Trop. Med. 2016;9:730–734. doi: 10.1016/j.apjtm.2016.06.012. [DOI] [PubMed] [Google Scholar]
- Pavlou M.G., Biesaga B., Touquet V., Lagal M., Balland A., Dufour, Hakimi M.A., Tardieux I. Toxoplasma parasite twisting motion mechanically induces host cell membrane fission to complete invasion within a protective vacuole. Cell. Host. Microbe. 2018;24:81–96. doi: 10.1016/j.chom.2018.06.003. [DOI] [PubMed] [Google Scholar]
- Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice E.C. Antioxidant activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free. Rad. Biol. Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Rivera F.N., Mondragón C.M., González P.S., Ramírez F.C., Mondragón C.T., Gómez de León C.K., Marrero P.Y., Arán V.J., Martins A.M., Mondragón F.R. A new type of quinoxalinone derivatives affects viability, invasion, and intracellular growth of Toxoplasma gondii tachyzoites in vitro. Parasitol. Res. 2016;115:2081–2096. doi: 10.1007/s00436-016-4953-1. [DOI] [PubMed] [Google Scholar]
- Rivera N., López C.P., Lemus M., Fortoul T., Reynalda D., Reyes A., Rivera E., Beltrán H., Malagón F. Antimalarial efficacy, cytotoxicity and genotoxicity of methanolic stem bark extract from Hintonia latiflora in a Plasmodium yoelii yoelii lethal murine malaria model. Parasitol. Res. 2014;113:1529–1536. doi: 10.1007/s00436-014-3797-9. [DOI] [PubMed] [Google Scholar]
- Shah B., Nayak B.S., Modi D.C. Terpenoids as A Potent Antiprotozoal Agents – A Review. Int. J. Pharm. Res. 2009 [Google Scholar]
- Smith A.R., Tejero D.D. Pleopeltis (polypodiaceae), a redefinition of the genus and nomenclatural novelties. Botan. Sci. 2014;92:43–58. [Google Scholar]
- Soeder R.W. Fern Constituents: including occurrence, chemotaxonomy and physiological activity. Bot. Rev. 1985;51:442–536. [Google Scholar]
- Tadesse A., Hymete A., Bekhit A., Mohammed S.F. Quantification of total polyphenols, catechin, caffeine, L-theanine, determination of antioxidant activity and effect on antileishmanial drugs of ethiopian tea leaves extracts. Pharmacognosy Res. 2015;7:S7–S14. doi: 10.4103/0974-8490.157991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Ven A.J., van de Ven E.M., Camps W.J., Melchers P.P., Koopmans J.W., van der Meer J.M. Anti-toxoplasma effect of pyrimethamine, trimethoprim and sulphonamides alone and in combination: implications for therapy. J. Antimicrob. Chemother. 1996;38:75–80. doi: 10.1093/jac/38.1.75. [DOI] [PubMed] [Google Scholar]
- Van Meerloo J., Kaspers G.J., Cloos J. Cell sensitivity assays: the MTT assay. Methods. Mol. Biol. 2011;731:237–245. doi: 10.1007/978-1-61779-080-5_20. [DOI] [PubMed] [Google Scholar]
- Vidal J.E. HIV-related cerebral toxoplasmosis revisited: current concepts and controversies of an old disease. J. Int. Assoc. Provid. AIDS. Care. 2019;18 doi: 10.1177/2325958219867315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner H.S., Bladt S., Zgainski E.M. A Thin Layer Chromatography Atlas. Spring-Verlang; Berlin Heidelberg New York Tokyo: 1984. Plant drug analysis. [Google Scholar]
- Weathers P.J., Elkholy S. Artemisinin: The Biosynthetic Pathway and Its Regulation in Artemisia annua, a Terpenoid-Rich Species. In. Vitro. Cell. Dev. Biol. Plant. 2006;42:309–317. [Google Scholar]
- Yadav R.N.S., Agarwala M. Phytochemical Analysis of Some Medicinal Plants. J. Phytol. 2011;3:10–14. [Google Scholar]
- Zacchino S.A., Butassi E., Cordisco E., Svetaz L.A. Hybrid combinations containing natural products and antimicrobial drugs that interfere with bacterial and fungal biofilms. Phytomed. 2017;37:14–26. doi: 10.1016/j.phymed.2017.10.021. [DOI] [PubMed] [Google Scholar]