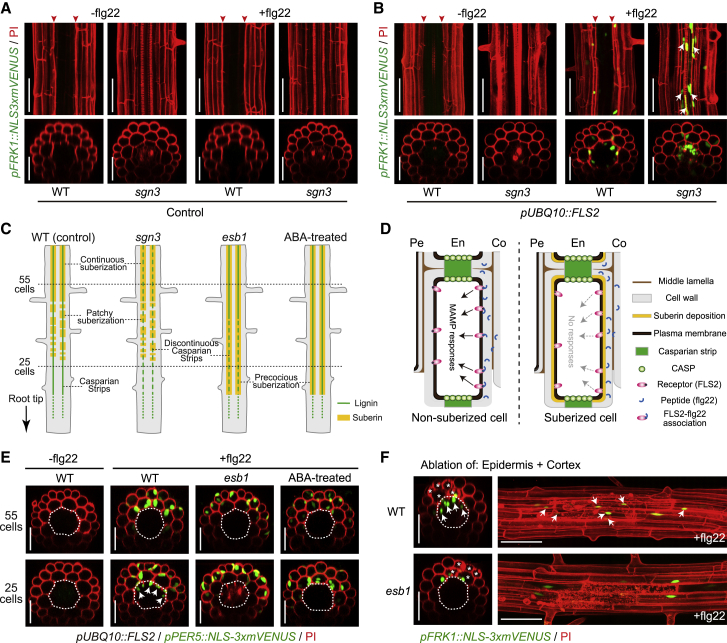
Figure 5.
Endodermal Barriers Compartmentalize MAMP Responses in Differentiated Roots
(A and B) Expression pattern of FRK1 marker in the absence or presence of flg22 in the differentiated zone of WT and endodermal barrier-defective sgn3-3 roots in Col-0 (A) and pUBQ10::FLS2 lines (B). Arrowheads indicate site of PI penetration block by the Casparian strips. Note the penetration of PI signals (red) into the stele in sgn3-3 mutants, revealing their barrier defects. Arrows in (B) indicate MAMP-responsive (FRK1-positive) nuclei (green) in the stele of sgn3-3. Maximal projections of confocal image stacks were taken at 25 endodermal cells after the onset of cell elongation. Nuclear-localized mVENUS signals (green) counterstained with PI. Scale bar, 50 μm.
(C) Schematic view of the two endodermal barriers—Casparian strips and suberin lamella—in different backgrounds (WT, sgn3-3, and esb1-1 mutants) and ABA treatment. Lignin and suberin deposition in the endodermis are represented by green and yellow lines, respectively.
(D) Schematic depicting the putative role of suberin lamellae in restricting receptor-peptide recognition on the cell surface. Primary stage and secondary stage of endodermal differentiation are presented by non-suberized (left) and suberized (right) endodermal cells, respectively. In non-suberized cells, peptides can access to the endodermal plasma membrane through apoplastic movement. The resulting plasma membrane-localized receptor-peptide (FLS2-flg22) association is capable of activating downstream MAMP responses inside the cell. By contrast, in suberized cells, direct MAMP signal perception on the cell surface is blocked by the presence of suberin lamellae between plasma membranes and primary cell walls of endodermal cells, interrupting the downstream responses.
(E) Representative images depicting expression of PER5 reporter combined with FLS2 constitutive expression line (pUBQ10::FLS2) in different backgrounds (WT and esb1-1 mutant) or pre-treatment with ABA (1 μM, 18 h). Dotted circles and arrows indicate the boundary between endodermal and cortical layers, and endodermal PER5 responsive nuclei, respectively. Scale bar, 50 μm.
(F) Co-ablation of epidermal and cortical cells triggers responsiveness to flg22 in differentiated endodermal cells of WT, but not in the precociously suberizing esb1-1 mutant. White asterisks indicate damaged cells by laser ablation. Maximal projections of confocal image stacks. Image overlays done as described for Figure 1D. Dotted circles and arrows indicate the boundary between endodermal and cortical layer, and endodermal FRK1 responsive nuclei, respectively. Scale bar, 50 μm.
See also Figure S6.