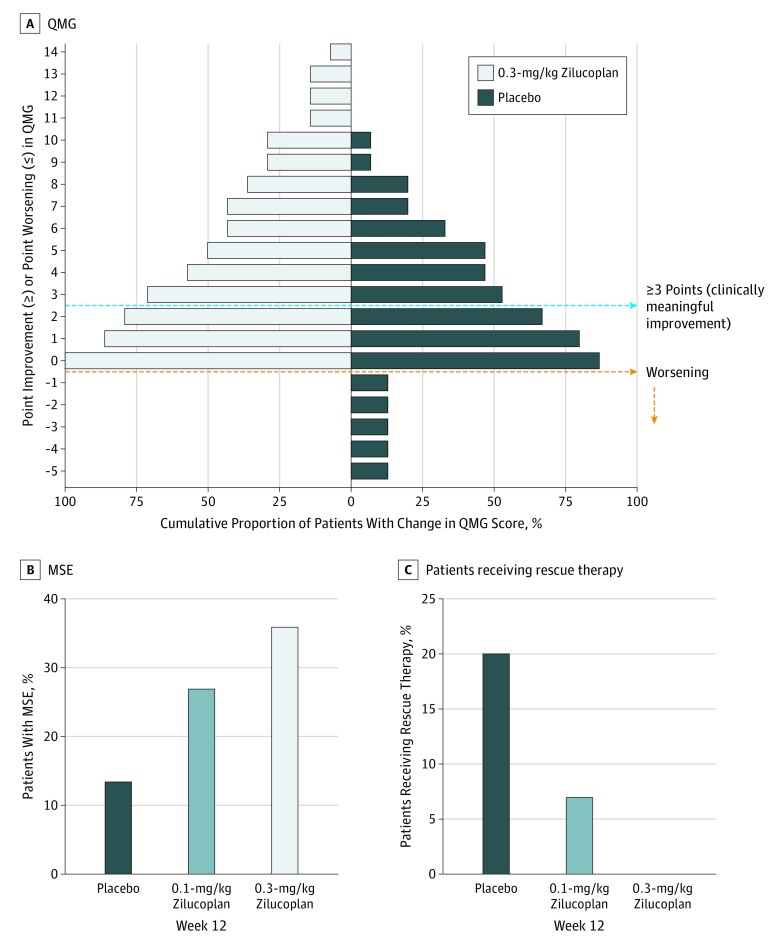
Figure 3. Categorical End Points.
A, Responder analysis for Quantitative Myasthenia Gravis (QMG) score with 0.3-mg/kg zilucoplan daily subcutaneously. B, Minimal symptom expression (MSE), defined as Myasthenia Gravis Activities of Daily Living score of 0 or 1 at 12 weeks. C, Patients who received treatment with rescue therapy with intravenous immunoglobulin or plasma exchange.