This noninferiority randomized clinical trial investigates whether dose reduction of biologics for stable psoriasis is noninferior to usual care in patients with plaque psoriasis.
Key Points
Question
Is a dose-reduction strategy of biologics noninferior to usual care in patients with stable psoriasis?
Findings
In this noninferiority randomized clinical trial of patients with plaque psoriasis, noninferiority could not be demonstrated for the Psoriasis Area and Severity Index at 12 months (dose reduction strategy vs usual care), although the mean Psoriasis Area and Severity Index difference was small. For the Dermatology Life Quality Index, noninferiority could be demonstrated, and dose tapering did not lead to more persistent flares.
Meaning
Dose reduction in real-life situations is possible, but a tight control scheme that monitors the Psoriasis Area and Severity Index and Dermatology Life Quality Index is warranted.
Abstract
Importance
Biologics revolutionized the treatment of psoriasis. Biologics are given in a fixed dose, but lower doses might be possible.
Objective
To investigate whether dose reduction (DR) of biologics in patients with stable psoriasis is noninferior to usual care (UC).
Design, Setting, and Participants
This pragmatic, open-label, prospective, controlled, noninferiority randomized clinical trial was conducted from March 1, 2016, to July 22, 2018, at 6 dermatology departments in the Netherlands. A total of 120 patients with plaque psoriasis and stable low disease activity who were receiving treatment with adalimumab, etanercept, or ustekinumab were studied.
Interventions
Patients were randomized 1:1 to DR (n = 60) or UC (n = 60). In the DR group, injection intervals were prolonged stepwise, leading to 67% and 50% of the original dose.
Main Outcomes and Measures
The primary outcome was between-group difference in disease activity corrected for baseline at 12 months compared with the predefined noninferiority margin of 0.5. Secondary outcomes were Psoriasis Area and Severity Index (PASI) score and health-related quality of life (including Dermatology Life Quality Index [DLQI] and Medical Outcomes Study 36-Item Short Form Health Survey scores), proportion of patients with short and persistent flares (defined as PASI and/or DLQI scores >5 for ≥3 months), and proportion of patients with successful dose tapering.
Results
Of 120 patients (mean [SD] age, 54.0 [13.2] years; 82 [68%] male), 2 patients were lost to follow-up, 2 patients had a protocol violation, and 5 patients had a protocol deviation, leaving 111 patients for the per-protocol analysis (53 in the DR group and 58 in the UC group). The median PASI scores at month 12 were 3.4 (interquartile range [IQR], 2.2-4.5) in the DR group and 2.1 (IQR, 0.6-3.6) in the UC group (mean difference, 1.2; 95% CI, 0.7-1.8). This indicates that noninferiority was not demonstrated for DR compared to UC. The median DLQI score at month 12 was 1.0 (IQR, 0.0-2.0) in the DR group and 0.0 (IQR, 0.0-2.0) in the UC group (mean difference, 0.8; 95% CI, 0.3-1.3), indicating noninferiority for DR compared with UC. No significant difference was found regarding persistent flares between groups (n = 5 in both groups). Twenty-eight patients (53%; 95% CI, 39%-67%) in the DR group tapered their dose successfully at 12 months. No severe adverse events related to the intervention occurred.
Conclusions and Relevance
In this trial, noninferiority was not demonstrated for DR of adalimumab, etanercept, and ustekinumab based on the PASI in patients with psoriasis compared with UC with the chosen noninferiority margin. However, the strategy was noninferior based on the DLQI. Dose tapering did not lead to persistent flares or safety issues.
Trial Registration
ClinicalTrials.gov Identifier: NCT02602925
Introduction
Psoriasis is an immune-mediated inflammatory skin disease for which several biologics are available. These drugs play an important role in the management of psoriasis, with adalimumab, etanercept, and ustekinumab being frequently used.1 However, long-term immunosuppressive effects of biologics may not be without risk, and biologics are expensive, placing a high financial burden on health care systems.2 For both reasons, aiming for the lowest effective dose is important.
Several studies3,4,5 have indicated that withdrawal of biologics frequently leads to quick relapse of psoriasis. However, dose reduction (DR) might lead to lower cumulative exposure without losing clinical efficacy in patients with psoriasis.6,7,8,9,10,11,12 These studies were not randomized and lacked a control group.
We hypothesized that when disease activity is monitored intensively while the biologic interval is prolonged stepwise, timely action can be taken to prevent persistent flares. Such a treatment strategy would allow physicians to determine safely the lowest effective dose for the individual patient. The aim of this trial was to investigate whether a disease activity–guided DR strategy is noninferior compared with usual care (UC) in patients with psoriasis.
Methods
Study Design and Participants
This pragmatic, open-label, noninferiority randomized clinical trial of adalimumab, etanercept, and ustekinumab was conducted from March 1, 2016, to July 22, 2018, at 1 academic and 5 regional Dutch hospitals. The study protocol is available in Supplement 1. Patients 18 years or older with plaque psoriasis and stable low disease activity using standard doses of adalimumab, etanercept, or ustekinumab for at least 6 months were included. We defined stable low disease activity as a Psoriasis Area and Severity Index (PASI) score of 5 or lower at 2 subsequent visits in the past 6 months and a Dermatology Life Quality Index (DLQI) score of 5 or lower at study inclusion.13 Exclusion criteria were a biologic indication other than psoriasis, concomitant use of systemic immunosuppressants, or severe comorbidities with short life expectancy. The study protocol and amendments were approved by the ethics committee of Radboud University Medical Center. All patients provided written informed consent. Data were deidentified, but a coding list was kept in a locked file separately; therefore, data were pseudonymized. The trial was performed in accordance with the Declaration of Helsinki14 and Good Clinical Practice guidelines. This trial followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline. The rationale and design are described elsewhere.13
Randomization and Procedures
In total, 120 patients were randomly assigned (1:1) to DR (n = 60) or UC (n = 60), and web-based randomization was performed with stratification by agent using variable permuted blocks with concealment allocation.15 All patients were seen once every 3 months. Dose reduction steps are described in the eMethods and eFigure 1 in Supplement 2. The PASI scores were assessed by treating physicians and research nurses with specific PASI training (S.A., J.M.P.A.v.d.R., L.J.v.V., M.D.N., J.M.M., P.M.O., M.I.K., M.A.B., and E.M.G.J.d.J.). No strict treatment rules were applied in the UC group, and in both groups, patients were allowed to continue using methotrexate and acitretin or start or stop topical therapy. To monitor disease activity and flares, the PASI and DLQI scores were assessed at study visits. We defined short disease flares as a single PASI score greater than 5 and/or a single DLQI score greater than 5. When short disease flares occurred, patients returned to their previous effective dose or original dose (eFigure 1 in Supplement 2). No further attempts for DR were made. Patients were instructed to contact their physician between visits if needed; this was followed by an additional visit or telephone contact within a week.
Outcomes
The primary outcome was between-group difference in disease activity (PASI) corrected for baseline PASI at 12 months compared with the noninferiority margin. Secondary outcomes were PASI score and health-related quality of life (including DLQI and Medical Outcomes Study 36-Item Short Form Health Survey [SF-36] scores), proportion of patients with short and persistent flares (we defined persistent flares as PASI and/or DLQI scores >5 for ≥3 months), and proportion of patients with successful dose tapering. Successful dose tapering was defined as patients with a lower dose than normal while maintaining PASI and DLQI scores of 5 or lower. Other secondary outcomes were indicators of successful dose tapering, treatment characteristics, level of C-reactive protein, severe adverse events (AEs), severe AEs related to the intervention, and AEs of special interest.
Statistical Analysis
Statistical analyses were performed according to the previously published analysis plan.13 Per-protocol analyses were performed because this type of analysis is the preferred and most conservative for noninferiority studies.16 Because no generally accepted noninferiority margin was available for absolute PASI, the noninferiority margin was set as one-third of the SD of PASI based on a rule of thumb for calculating standardized effect sizes (difference between groups = standardized effect size × pooled population SD).17 The standardized effect size was set at 0.3.18 The SD of PASI in the BioCapture registry19,20 was 1.4, leading to a margin of 0.5. Because we performed our analysis with correction for the baseline PASI score, a formula that increases power significantly was used to account for the correlation between baseline and follow-up PASI scores.21 This correlation was 0.67 in the BioCapture registry. When performing an unpaired, 2-tailed t test for sample size calculation, the sample size could be multiplied by 1 − ρ2 after adding 1 extra person per group (ρ is the correlation between the baseline measure and the follow-up measure). Thus, when performing an analysis of covariance corrected for baseline PASI score with the noninferiority margin set at 0.5, α = .05, and ρ = 0.67, we achieved 80% power when enrolling 54 patients per arm. To account for a 10% rate of missing individuals, 60 patients were included per arm.
For the primary outcome, an additional intention-to-treat analysis was performed. Patients who deviated from the protocol were included for the intention-to-treat analysis. The primary outcome of PASI score at 12 months was corrected for the baseline PASI score using a general linear model (analysis of covariance). Noninferiority was assumed if the upper limit of the 95% CI of this difference did not exceed the noninferiority margin.
For all outcome measures (PASI, DLQI, and SF-36), descriptive statistics were computed at all time points, and scores were, per time point, compared between the treatment groups. To gain insight into the differences in the course of these outcomes between groups, we decided ad hoc to perform additional linear mixed-model analyses (eMethods in Supplement 2). For the DLQI, we performed a prespecified noninferiority analysis at 12 months with a predefined noninferiority margin that was based on the rule of thumb (one-third SD) and clinical relevance. An SD of 6 on the DLQI for patients from the BioCapture registry led to a noninferiority margin of 2.13 Proportions of patients with short and persistent flares were compared between groups using the Fisher exact test.
The proportion of patients with successful dose tapering was calculated. Indicators of successful dose tapering, expressed as relative risks, were analyzed with a log binomial regression model. Possible indicators of successful dose tapering were selected a priori based on clinical relevance and the literature. When more than 1 variable in univariable log binomial regression analysis produced a P < .20, a multivariable log binomial regression analysis was planned. A backward selection procedure was used based on P < .05 to arrive at a final model. Because the log binomial model was unable to fit in a multivariable setting, PASI score as the continuous variable was recoded into 2 categories: higher than 3.0 and 3.0 or lower.
Baseline characteristics and treatment characteristics were analyzed using descriptive statistics. For nominal variables, numbers (percentages) are presented, and for continuous variables, means (SDs) or medians (interquartile ranges [IQRs]) are presented depending on skewness of the data. The mean cumulative dose per patient for each biologic throughout the study was calculated. The percentage of the dose used in DR compared with UC was calculated.
The severe AEs were described per group with corresponding monthly event rates and rate ratios to compare the groups. All AEs and their association with dose tapering were assessed by 2 reviewers (S.A., J.M.P.A.v.d.R.). We decided ad hoc to perform additional analyses on AEs of special interest, including musculoskeletal concerns, given that a substantial number of patients also had psoriatic arthritis. All musculoskeletal AEs were checked by a rheumatologist (A.A.d.B.) as a third reviewer. Two classifications were made: evident psoriatic arthritis (exacerbations) and all musculoskeletal concerns.
Statistical analyses were performed with SPSS software, version 23.0 (SPSS Inc) and SAS software, version 9.4 (SAS Institute Inc).
Results
Patients
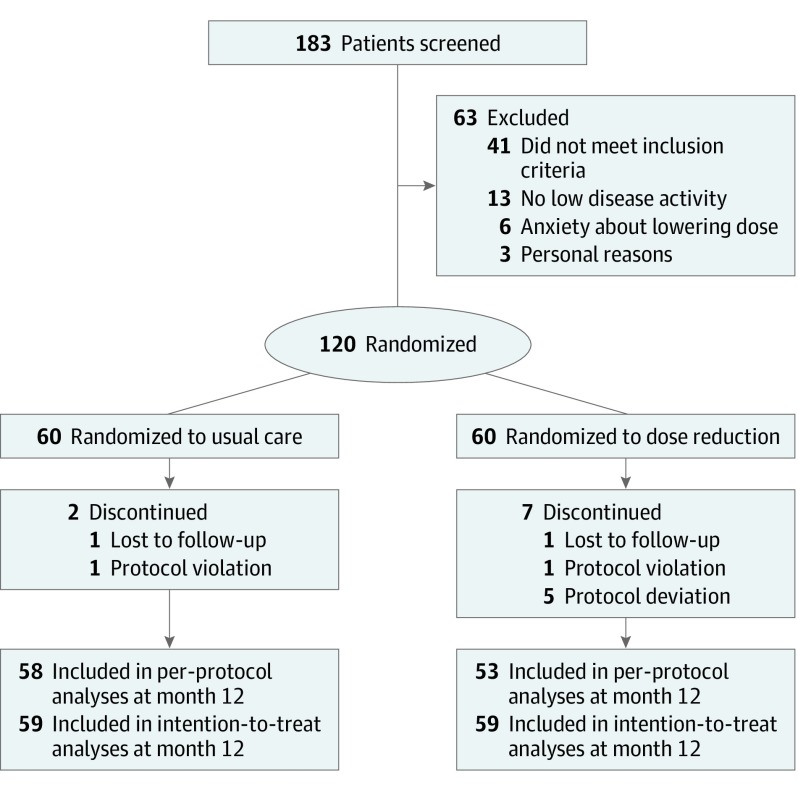
In total, 120 patients were included in the study (mean [SD] age, 54.0 [13.2] years; 82 [68%] male), with 60 assigned to DR and 60 to UC (Figure 1). Two patients were lost to follow-up, 2 patients had a protocol violation, and 5 patients had a protocol deviation, leaving 111 patients for the per-protocol analysis (53 in the DR group and 58 in the UC group). Of the 2 patients lost to follow-up, one was lost to follow-up because of psychiatric illness and the other patient continued treatment in a nonparticipating hospital. Demographic and baseline characteristics were well balanced in the DR and UC groups (Table 1). These characteristics mimic a typical cohort of psoriasis patients taking biologics except for low disease activity at baseline. The number of missing visits was small (1% of all planned visits [1% for PASI, 2% for DLQI, and 6% for SF-36]), and multiple imputation was deemed to be unnecessary.
Figure 1. Study Design and Patient Disposition.
Table 1. Baseline Characteristics of the Study Patientsa.
| Characteristic | Usual Care (n = 60) | Dose Reduction (n = 60) |
|---|---|---|
| Male | 42 (70) | 40 (67) |
| Age, mean, (SD), y | 57 (13.3) | 53 (12.9) |
| Onset of psoriasis, mean (SD), y | 28 (14.0) | 24 (11.4) |
| Disease duration, mean (SD), y | 28 (12.3) | 28 (12.9) |
| Biologic duration, mean (SD), y | 4.8 (2.9) | 4 (2.8) |
| Psoriatic arthritis | 12 (20) | 19 (32) |
| BMI, mean (SD) | 28 (4.9) | 29 (5.4) |
| Weight, mean (SD), kg | 87 (17.0) | 86 (18.3) |
| Current alcohol use | 45 (75) | 44 (73) |
| Current smoking | 12 (20) | 13 (22) |
| Past smoking | 29 (48) | 32 (53) |
| Disease activity | ||
| PASI score, median (IQR) | 1.3 (0.3-2.7) | 1.8 (0.6-2.8) |
| DLQI score, median (IQR) | 0.0 (0-2) | 0.0 (0-1) |
| C-reactive protein level, mean (SD), mg/L | 2.8 (5.3) | 2.2 (1.9) |
| Medical history | ||
| Diabetes (types 1 and 2) | 10 (17) | 7 (12) |
| Hypertension | 23 (38) | 20 (33) |
| Hypercholesterolemia | 20 (33) | 16 (27) |
| Myocardial infarction | 4 (7) | 3 (5) |
| Cerebrovascular incident | 4 (7) | 4 (7) |
| Malignant tumor | 5 (8) | 2 (3) |
| NMSC | 5 (8) | 2 (3) |
| IBD | 1 (2) | 0 |
| Rheumatologic condition | 11 (18) | 10 (17) |
| Treatment in the study | ||
| Adalimumab | 27 (45) | 25 (42) |
| Etanercept | 14 (23) | 14 (23) |
| Ustekinumab | 19 (32) | 21 (35) |
| Methotrexate or acitretin | 4 (7) | 4 (7) |
| Previous treatments | ||
| Topical corticosteroids | 60 (100) | 60 (100) |
| Dithranol | 29 (48) | 28 (47) |
| UV therapy (UV-B or PUVA) | 57 (95) | 57 (95) |
| Retinoid (acitretin) | 27 (45) | 27 (45) |
| Fumaric acid esters | 29 (48) | 30 (50) |
| Cyclosporine | 31 (52) | 22 (32) |
| Methotrexate | 54 (90) | 55 (92) |
| Previous biologics | 31 (52) | 30 (50) |
| Adalimumab | 14 (23) | 19 (32) |
| Etanercept | 22 (37) | 19 (32) |
| Ustekinumab | 4 (7) | 3 (5) |
| Secukinumab | 0 | 0 |
| Infliximab | 4 (7) | 0 |
| Alefacept | 5 (8) | 3 (5) |
| Other biologics | 5 (8) | 9 (15) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); DLQI, Dermatology Life Quality Index; IBD, inflammatory bowel disease; IQR, interquartile range; NMSC, nonmelanoma skin cancer; PASI, Psoriasis Area and Severity Index; PUVA, psoralen-UV-A.
SI conversion factor: To convert C-reactive protein to nanomoles per liter, multiply by 9.524.
Data are presented as number (percentage) of patients unless otherwise indicated.
Primary Outcome
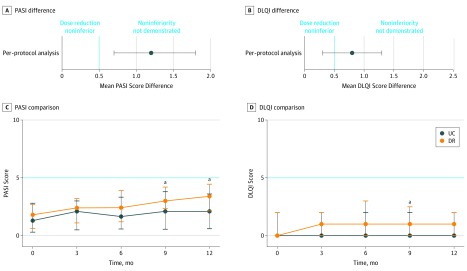
The per-protocol analysis found a median PASI score at month 12 of 3.4 (IQR, 2.2-4.5) in the DR group and 2.1 (IQR, 0.6-3.6) in the UC group. The mean difference in the PASI score at month 12, corrected for baseline PASI score, was 1.2 (95% CI, 0.7-1.8). The lower limit of the 95% CI exceeded the noninferiority margin by 0.5 (Figure 2A). The intention-to-treat analysis found similar results; the mean difference in PASI score at month 12, corrected for baseline PASI score, was 1.1 (95% CI, 0.6-1.7).
Figure 2. Primary and Secondary Outcome Analyses .
A, Mean difference in the Psoriasis Area and Severity Index (PASI) scores between the usual care (UC) and dose reduction (DR) groups after 12 months. B, Mean difference in the Dermatology Life Quality Index (DLQI) scores between the DR and UC groups after 12 months. C, Comparison of the PASI scores between the UC and DR groups. The PASI scores are depicted at months 0, 3, 6, 9, and 12. D, Comparison of the DLQI scores between the UC and DR groups at months 0, 3, 6, 9, and 12. Errors bars indicate 95% CIs. The dotted vertical line depicts the noninferiority margin in context of the PASI and DLQI difference.
aP < .05.
Secondary Outcomes
Disease activity (PASI) was compared at all time points between the UC and DR groups (Figure 2B). A significant difference was seen between the UC and DR scores at 9 months (mean UC score, 2.3; 95% CI, 1.8-2.9; mean DR score, 3.4; 95% CI, 2.8-3.9; P = .003) and 12 months (mean UC score, 2.2; 95% CI, 1.8-2.6; mean DR score, 3.4; 95% CI, 3.0-3.9; P = .001). The PASI linear mixed model is presented in eFigure 2A and eTable 1 in Supplement 2.
The median DLQI score at month 12 was 1.0 (IQR, 0.0-2.0) for the DR group and 0.0 (IQR, 0.0-2.0) for the UC group. The mean difference for the DLQI score at month 12, corrected for baseline DLQI score, was 0.8 (95% CI, 0.3-1.3). The upper limit of the 95% CI for the mean difference did not cross the noninferiority margin of 2.0 (Figure 2C), indicating noninferiority for DR for DLQI. The median DLQI scores were compared at all time points (Figure 2D). A significant difference was observed only at 9 months (mean DLQI score in UC group, 1.4; 95% CI, 0.7-2.1; mean DLQI score in DR group, 1.6; 95% CI, 1.1- 2.1; P = .01). Linear mixed models for the DLQI and SF-36 are presented in eFigure 2B-D and eTable 1 in Supplement 2. A significant difference regarding short flares was found between the DR and UC groups during the study (19 of 53 patients [36%; 95% CI, 24%-50%] in the DR group and 8 of 58 patients [14%; 95% CI, 7%-26%] in the UC group; P = .04). No significant difference regarding persistent flares was found between the DR and UC groups during the study period (5 of 53 patients [9%; 95% CI, 4%-21%] in the DR group and 5 of 58 patients [9%; 95% CI, 3%-20%] in the UC group; P > .99). The relative risk was 1.1 (95% CI, 0.3-3.6) in the DR vs UC group (P > .99). Time until persistent flares is shown in eFigure 3 in Supplement 2. Four patients in the DR group with persistent flares did not have PASI and DLQI scores that returned to 5 or lower after 12 months. In the UC group, 1 patient did not have PASI and DLQI scores that returned to 5 or lower after 12 months. In both groups, no switch to another biologic therapy was required. Eighteen additional visits were performed in the DR group vs 1 in the UC group.
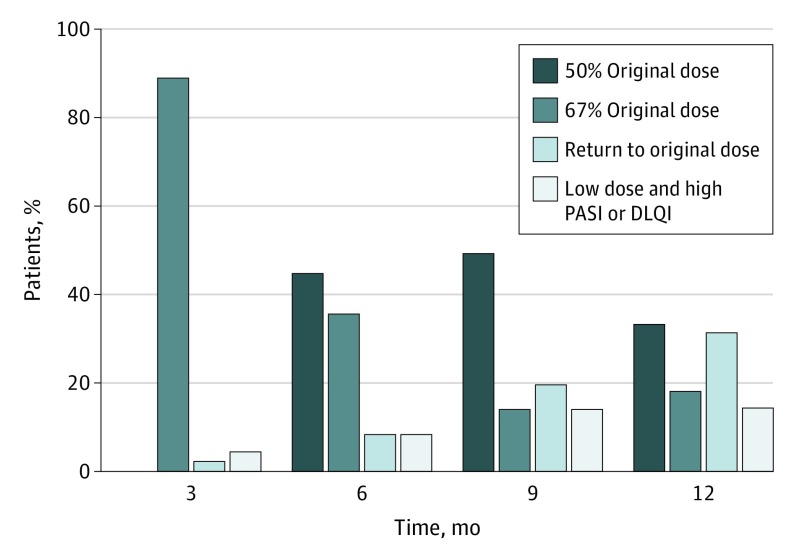
Twenty-eight patients (53%; 95% CI, 39%-67%) tapered their dose successfully after 12 months. Ten patients (19%; 95% CI, 10%-32%) were taking two-thirds of their original dose, and 18 patients (34%; 95% CI, 22%-48%) were taking half of their original dose (Figure 3). The proportion of patients with successful DR split per biologic is given in eTable 2 in Supplement 2. Thirty-six patients (68%; 95% CI, 54%-80%) used a lower dose until their 12-month visit, but 8 of the DRs (15%; 95% CI, 7%-28%) were not deemed to be successful because of a PASI or DLQI score higher than 5 at that time. No variables were associated with successful DR in the final model (eTable 3 in Supplement 2).
Figure 3. Proportions of Patients With Successful Dose Tapering on Specific Dosages.
DLQI indicates Dermatology Life Quality Index; PASI, Psoriasis Area and Severity Index.
Treatment Characteristics
The mean cumulative doses during 12 months per patient for each biologic are given in eTable 4 in Supplement 2. The percentage DR varied between 23% and 34%. A significant difference was seen in topical corticosteroid use between both groups. In the DR group, 44 patients (73%; 95% CI, 60%-84%) used topical steroids for a mean of 87 days (95% CI, 65-109 days), and in the UC group, 21 patients (35%; 95% CI, 23%-48%) used topical steroids for a mean of 35 days (95% CI, 20-50 days) in 12 months (P < .001). Rates of additional methotrexate or acitretin use were 13% in the DR group and 12% in the UC group at baseline. During the study, the number of patients using methotrexate or acitretin (including doses) remained stable.
Safety
Six patients (11%; 95% CI, 5%-24%) in the DR group and 6 patients (10%; 95% CI, 4%-22%) in the UC group reported at least 1 severe AE (P > .99). None of these severe AEs were assumed to be causally related to DR. One patient had 5 severe AEs because of preexistent peripheral arterial disease. Relevant AEs and severe AEs and corresponding rates and rate ratios are given in Table 2. No significant differences in AEs were seen in either group except for general musculoskeletal concerns, which occurred more often in the DR group (rate ratio, 4.92; 95% CI, 2.04-11.87; P < .001). In each group, 1 patient presented with evident psoriatic arthritis concerns during the study, and both patients had been diagnosed as having psoriatic arthritis before study inclusion. Adverse events are described in the eResults in Supplement 2.
Table 2. Safety of Dose Reduction vs Usual Care.
| Variable | Event Rate per Month (95% CI) | RR (95% CI) for Dose Reduction vs Usual Care | P Value | |
|---|---|---|---|---|
| Usual Care (n = 58) | Dose Reduction (n = 53) | |||
| SAEs | ||||
| All | 0.010 (0.005-0.021)a | 0.015 (0.008-0.028)b | 1.51 (0.57-4.00) | .41 |
| Related to interventionc | 0 | 0 | NA | NA |
| All AEs | 0.186 (0.157-0.221) | 0.216 (0.183-0.254) | 1.16 (0.91-1.47) | .23 |
| AEs of special interest per category | ||||
| Infectious event | 0.103 (0.082-0.130) | 0.104 (0.082-0.132) | 1.01 (0.73-1.40) | .95 |
| Cardiovascular event | 0.004 (0.001-0.013) | 0.005 (0.001-0.014) | 1.05 (0.21-5.22) | .95 |
| Malignant tumor | 0 | 0.002 (0.000-0.011) | NA | NA |
| Nonmelanoma skin cancer | 0.001 (0.000-0.010) | 0.002 (0.000-0.011) | 1.10 (0.07-16.84) | .97 |
| Musculoskeletal events | 0.009 (0.004-0.019) | 0.042 (0.029-0.060) | 4.92 (2.04-11.87) | <.001 |
| Psoriatic arthritis exacerbation | 0.0014 (0.0002-0.0100) | 0.0015 (0.0002-0.0106) | 0.95 (0.059-15.15) | .97 |
| Skin events | 0.014 (0.008-0.026) | 0.006 (0.002-0.016) | 0.42 (0.13-1.34) | .14 |
| Elective surgery | 0.006 (0.002-0.015) | 0.009 (0.004-0.020) | 1.60 (0.45-5.60) | .48 |
| Death | 0 | 0 | NA | NA |
Abbreviations: AE, adverse event; NA, not applicable; RR, rate ratio; SAE, severe adverse event.
One patient in the usual care group had 2 SAEs.
One patient in the dose reduction group had 5 SAEs from a preexistent disease.
All SAEs were deemed unrelated to the intervention of dose tapering.
Discussion
On the basis of the examined DR strategy, noninferiority of the PASI score at 12 months was not demonstrated for DR of adalimumab, etanercept, and ustekinumab in patients with psoriasis compared with UC. The mean difference in the PASI score between groups was 1.2 (95% CI, 0.7-1.8) and was larger than the noninferiority margin of 0.5. However, noninferiority of the DLQI was demonstrated. The mean difference in the DLQI score between both groups at 12 months was 0.8 (95% CI, 0.3-1.3), which did not exceed the predefined noninferiority margin of 2.0. No significant differences were found regarding persistent flares. No severe AEs related to intervention and no differences in quality of life between groups were seen. Successful DR was possible in 53% (95% CI, 39%-67%) of patients. The mean cumulative dose for DR was 34% lower compared with the UC dose in patients taking adalimumab, 26% lower in patients taking etanercept, 23% lower in patients taking 45 mg of ustekinumab, and 34% lower in patients taking 90 mg of ustekinumab.
As stated, noninferiority could not be demonstrated for the primary end point. We found a mean difference in PASI score of 1.2, which exceeded the noninferiority margin. This finding means that physicians and patients need to be aware of the chance of an increase in PASI score when trying to reduce the dose. It is important to weigh the risk of a PASI score increase against the benefits of using a lower dose of the biologic. A significant increase in nonspecific musculoskeletal concerns in the DR group compared with the UC group was reported. These concerns were mainly mild, and evident (exacerbation of) psoriatic arthritis was seen once in both the DR and UC groups. Because of the open-label design of this study, reporting bias could be present for these concerns. Patients in the DR group used more topical treatment than those in the UC group, which was inherent to the DR strategy.
For psoriasis, noncomparative, observational studies6,10,11,12,22,23 have been conducted with interval prolongation, showing success rates that range from 22% to 90%. Our study found successful DR in 53% of patients. Differences that were observed with other studies6,10,11,12,22,23 can be explained by study design, population, and the tight control character of our strategy. The present strategy was based on prevailing treatment goals, which defined low disease activity as PASI and DLQI scores of 5 or lower.24 However, current treatment goals are shifting toward a PASI score of 3 or lower.25 In our multivariate regression model, we analyzed the threshold PASI score of 3 or lower vs higher than 3 as well, which was not identified as an indicator of successful DR.
Strengths and Limitations
A strength of our study was the high internal validity by means of the randomized design, use of validated outcome measures, and good data integrity. The pragmatic design led to a high external validity of our results. The number of patients needed to achieve enough statistical power was met, and missing data were scarce. The risk of selection bias or confounding was deemed to be low because both groups seemed to be balanced in baseline characteristics. Incorporation of the DLQI in the tapering strategy gave important insight from the perspective of the patient.
The open-label design might be regarded as a limitation. Patients and physicians could return to using a higher dose, assuming that patients generally fear a flare. However, few patients returned at their own initiative to higher (n = 4) or original (n = 2) doses. Moreover, the goal of this pragmatic study was to provide high external validity, and blinding would have lowered external validity. An absolute PASI score was used instead of a relative PASI or Psoriasis Global Assessment score. By design, patients had low disease activity, and a PASI score of 75 of 90 is almost unachievable with a starting PASI score lower than 5. Dose reduction was accompanied by higher topical treatment use and more visits. Tumor necrosis factor and interleukin 12 and 23 blockers were analyzed as a group because the focus was on the DR strategy and not on the working mechanism itself. Thus, for per-drug conclusions, this study was underpowered but still provides relevant pilot data. Another limitation is the absence of a validated flare criterion for psoriasis. Our flare criterion is therefore a definition based on expert opinion and combined with a patient-reported outcome (DLQI). Both DLQI and PASI weighed equally on the algorithm of clinical decision-making in case a flare occurred.
Conclusions
In this first, to our knowledge, randomized, tightly controlled noninferiority trial, a DR strategy for adalimumab, etanercept, and ustekinumab in patients with psoriasis, noninferiority of DR compared with standard dose was not demonstrated for PASI at 12 months. Nevertheless, many patients were able to prolong the interval of their biologic use while maintaining low PASI and DLQI scores. The cumulative DR varied from 23% to 34% depending on the biologic. In 53% of patients, dose was successfully tapered, and no severe AEs related to the intervention were observed. No difference regarding persistent flares was found in either group. This disease activity–guided DR strategy is expected to have an effect on health care expenditures.
Trial Protocol
eMethods. Detailed Methods
eFigure 1. Study Population and Decision Steps
eFigure 2. Linear Mixed Model Analyses PASI, DLQI and SF-36
eFigure 3. Kaplan-Meier Curve Presenting Time Until Persistent Flare in the Usual Care and Dose Reduction Group
eTable 1. Subgroup Analyses: Outcome Measures at Baseline and End of Study Month
eTable 2. Proportions of Patients With Dosing Steps After 12 Months
eTable 3. Factors Associated With Failure of Dose Tapering
eTable 4. Cumulative Dose Per Patients in 12 Months
eResults. Adverse Events
Data Sharing Statement
References
- 1.Conrad C, Gilliet M. Psoriasis: from pathogenesis to targeted therapies. Clin Rev Allergy Immunol. 2018;54(1):102-113. doi: 10.1007/s12016-018-8668-1 [DOI] [PubMed] [Google Scholar]
- 2.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295(19):2275-2285. doi: 10.1001/jama.295.19.2275 [DOI] [PubMed] [Google Scholar]
- 3.Papp K, Menter A, Poulin Y, Gu Y, Sasso EH. Long-term outcomes of interruption and retreatment vs. continuous therapy with adalimumab for psoriasis: subanalysis of REVEAL and the open-label extension study. J Eur Acad Dermatol Venereol. 2013;27(5):634-642. doi: 10.1111/j.1468-3083.2012.04515.x [DOI] [PubMed] [Google Scholar]
- 4.Gordon KB, Gottlieb AB, Langely RG, et al. Adalimumab retreatment successfully restores clinical response and health-related quality of life in patients with moderate to severe psoriasis who undergo therapy interruption. J Eur Acad Dermatol Venereol. 2015;29(4):767-776. doi: 10.1111/jdv.12677 [DOI] [PubMed] [Google Scholar]
- 5.Chiu HY, Hui RC, Tsai TF, et al. Predictors of time to relapse following ustekinumab withdrawal in patients with psoriasis who had responded to therapy: an eight-year multicenter study [published online January 28, 2019]. J Am Acad Dermatol. doi: 10.1016/j.jaad.2019.01.035 [DOI] [PubMed] [Google Scholar]
- 6.Taniguchi T, Noda S, Takahashi N, Yoshimura H, Mizuno K, Adachi M. An observational, prospective study of monthly adalimumab therapy for disease maintenance in psoriasis patients: a possible new therapeutic option for good responders to the initial induction treatment. J Eur Acad Dermatol Venereol. 2013;27(11):1444-1447. doi: 10.1111/j.1468-3083.2012.04610.x [DOI] [PubMed] [Google Scholar]
- 7.Na JI, Kim JH, Park KC, Youn SW. Low-dose etanercept therapy in moderate to severe psoriasis in Korean. J Dermatol. 2008;35(8):484-490. doi: 10.1111/j.1346-8138.2008.00508.x [DOI] [PubMed] [Google Scholar]
- 8.Fotiadou C, Lazaridou E, Sotiriou E, Ioannides D. Adalimumab for psoriasis in Greece: clinical experience in a tertiary referral centre. J Eur Acad Dermatol Venereol. 2012;26(10):1298-1303. doi: 10.1111/j.1468-3083.2011.04290.x [DOI] [PubMed] [Google Scholar]
- 9.López-Ferrer A, Vilarrasa E, Gich IJ, Puig L. Adalimumab for the treatment of psoriasis in real life: a retrospective cohort of 119 patients at a single Spanish centre. Br J Dermatol. 2013;169(5):1141-1147. doi: 10.1111/bjd.12543 [DOI] [PubMed] [Google Scholar]
- 10.van Bezooijen JS, van Doorn MBA, Schreurs MWJ, et al. Prolongation of biologic dosing intervals in patients with stable psoriasis: a feasibility study. Ther Drug Monit. 2017;39(4):379-386. doi: 10.1097/FTD.0000000000000420 [DOI] [PubMed] [Google Scholar]
- 11.Baniandrés O, Rodríguez-Soria VJ, Romero-Jiménez RM, Suárez R. Dose modification in biologic therapy for moderate to severe psoriasis: a descriptive analysis in a clinical practice setting. Actas Dermosifiliogr. 2015;106(7):569-577. doi: 10.1016/j.adengl.2015.06.004 [DOI] [PubMed] [Google Scholar]
- 12.Rodrigo-Nicolas BG-CE, Quecedo-Estebanez E. Adalimumab dose reduction in psoriasis: results in a series of 12 patients [abstract]. J Am Acad Dermatol. 2014;70(5)(suppl 1):AB164. doi: 10.1016/j.jaad.2014.01.681 [DOI] [Google Scholar]
- 13.Atalay S, van den Reek JMPA, van Vugt LJ, et al. Tight controlled dose reduction of biologics in psoriasis patients with low disease activity: a randomized pragmatic non-inferiority trial. BMC Dermatol. 2017;17(1):6. doi: 10.1186/s12895-017-0057-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 15.Castor website. https://www.castoredc.com/. Accessed September 15, 2019.
- 16.Soonawala D, Dekkers OM. ‘Non-inferiority’ trials: tips for the critical reader: research methodology 3 [in Dutch]. Ned Tijdschr Geneeskd. 2012;156(19):A4665. [PubMed] [Google Scholar]
- 17.Cook JA, Hislop J, Adewuyi TE, et al. Assessing methods to specify the target difference for a randomised controlled trial: DELTA (Difference ELicitation in TriAls) review. Health Technol Assess. 2014;18(28):v-vi, 1-175. doi: 10.3310/hta18280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 19.Zweegers J, Groenewoud JMM, van den Reek JMPA, et al. Comparison of the 1- and 5-year effectiveness of adalimumab, etanercept and ustekinumab in patients with psoriasis in daily clinical practice: results from the prospective BioCAPTURE registry. Br J Dermatol. 2017;176(4):1001-1009. doi: 10.1111/bjd.15023 [DOI] [PubMed] [Google Scholar]
- 20.Zweegers J, Roosenboom B, van de Kerkhof PC, et al. Frequency and predictors of a high clinical response in patients with psoriasis on biological therapy in daily practice: results from the prospective, multicenter BioCAPTURE cohort. Br J Dermatol. 2017;176(3):786-793. doi: 10.1111/bjd.14888 [DOI] [PubMed] [Google Scholar]
- 21.Borm GF, Fransen J, Lemmens WA. A simple sample size formula for analysis of covariance in randomized clinical trials. J Clin Epidemiol. 2007;60(12):1234-1238. doi: 10.1016/j.jclinepi.2007.02.006 [DOI] [PubMed] [Google Scholar]
- 22.Romero-Jimenez RM, Escudero-Vilaplana V, Baniandres Rodriguez O, García Martín E, Mateos Mayo A, Sanjurjo Saez M. Association between clinical factors and dose modification strategies in the treatment with ustekinumab for moderate-to-severe plaque psoriasis. J Dermatolog Treat. 2018;29(8):792-796. doi: 10.1080/09546634.2018.1466978 [DOI] [PubMed] [Google Scholar]
- 23.Blauvelt A, Ferris LK, Yamauchi PS, et al. Extension of ustekinumab maintenance dosing interval in moderate-to-severe psoriasis: results of a phase IIIb, randomized, double-blinded, active-controlled, multicentre study (PSTELLAR). Br J Dermatol. 2017;177(6):1552-1561. doi: 10.1111/bjd.15722 [DOI] [PubMed] [Google Scholar]
- 24.Mrowietz U, Kragballe K, Reich K, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res. 2011;303(1):1-10. doi: 10.1007/s00403-010-1080-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carretero G, Puig L, Carrascosa JM, et al. ; from the Spanish Group of Psoriasis . Redefining the therapeutic objective in psoriatic patients candidates for biological therapy. J Dermatolog Treat. 2018;29(4):334-346. doi: 10.1080/09546634.2017.1395794 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods. Detailed Methods
eFigure 1. Study Population and Decision Steps
eFigure 2. Linear Mixed Model Analyses PASI, DLQI and SF-36
eFigure 3. Kaplan-Meier Curve Presenting Time Until Persistent Flare in the Usual Care and Dose Reduction Group
eTable 1. Subgroup Analyses: Outcome Measures at Baseline and End of Study Month
eTable 2. Proportions of Patients With Dosing Steps After 12 Months
eTable 3. Factors Associated With Failure of Dose Tapering
eTable 4. Cumulative Dose Per Patients in 12 Months
eResults. Adverse Events
Data Sharing Statement