This cohort study assesses cardiovascular functional reserve before and after kidney transplant in patients with end-stage renal disease.
Key Points
Question
How is kidney transplant associated with cardiovascular functional reserve?
Findings
In this cohort study of 166 patients with stage 5 chronic kidney disease and 87 patients with hypertension only to assess change to cardiovascular functional reserve after improving the uremic milieu through a kidney transplant using state-of-the-art cardiopulmonary exercise testing, improved cardiovascular functional reserve was seen 1 year after kidney transplant in the absence of significant alterations in left ventricular morphologic findings.
Meaning
Improved cardiovascular reserve after kidney transplant may be associated with ultrastructural and functional alterations to the cardiovascular system and may not be associated with a change in left ventricular muscle mass.
Abstract
Importance
Restitution of kidney function by transplant confers a survival benefit in patients with end-stage renal disease. Investigations of mechanisms involved in improved cardiovascular survival have relied heavily on static measures from echocardiography or cardiac magnetic resonance imaging and have provided conflicting results to date.
Objectives
To evaluate cardiovascular functional reserve in patients with end-stage renal disease before and after kidney transplant and to assess functional and morphologic alterations of structural-functional dynamics in this population.
Design, Setting, and Participants
This prospective, nonrandomized, single-center, 3-arm, controlled cohort study, the Cardiopulmonary Exercise Testing in Renal Failure and After Kidney Transplantation (CAPER) study, included patients with stage 5 chronic kidney disease (CKD) who underwent kidney transplant (KTR group), patients with stage 5 CKD who were wait-listed and had not undergone transplant (NTWC group), and patients with hypertension only (HTC group) seen at a single center from April 1, 2010, to January 1, 2013. Patients were followed up longitudinally for up to 1 year after kidney transplant. Clinical data collection was completed February 2014. Data analysis was performed from June 1, 2014, to March 5, 2015. Further analysis on baseline and prospective data was performed from June 1, 2017, to July 31, 2019.
Main Outcomes and Measures
Cardiovascular functional reserve was objectively quantified using state-of-the-art cardiopulmonary exercise testing in parallel with transthoracic echocardiography.
Results
Of the 253 study participants (mean [SD] age, 48.5 [12.7] years; 141 [55.7%] male), 81 were in the KTR group, 85 in the NTWC group, and 87 in the HTC group. At baseline, mean (SD) maximum oxygen consumption (V̇O2max) was significantly lower in the CKD groups (KTR, 20.7 [5.8] mL · min−1 · kg−1; NTWC, 18.9 [4.7] mL · min−1 · kg−1) compared with the HTC group (24.9 [7.1] mL · min−1 · kg−1) (P < .001). Mean (SD) cardiac left ventricular mass index was higher in patients with CKD (KTR group, 104.9 [36.1] g/m2; NTWC group, 113.8 [37.7] g/m2) compared with the HTC group (87.8 [16.9] g/m2), (P < .001). Mean (SD) left ventricular ejection fraction was significantly lower in the patients with CKD (KTR group, 60.1% [8.6%]; NTWC group, 61.4% [8.9%]) compared with the HTC group (66.1% [5.9%]) (P < .001). Kidney transplant was associated with a significant improvement in V̇O2max in the KTR group at 12 months (22.5 [6.3] mL · min−1 · kg−1; P < .001), but the value did not reach the V̇O2max in the HTC group (26.0 [7.1] mL · min−1 · kg−1) at 12 months. V̇O2max decreased in the NTWC group at 12 months compared with baseline (17.7 [4.1] mL · min−1 · kg−1, P < .001). Compared with the KTR group (63.2% [6.8%], P = .02) or the NTWC group (59.3% [7.6%], P = .003) at baseline, transplant was significantly associated with improved left ventricular ejection fraction at 12 months but not with left ventricular mass index.
Conclusions and Relevance
The findings suggest that kidney transplant is associated with improved cardiovascular functional reserve after 1 year. In addition, cardiopulmonary exercise testing was sensitive enough to detect a decline in cardiovascular functional reserve in wait-listed patients with CKD. Improved V̇O2max may in part be independent from structural alterations of the heart and depend more on ultrastructural changes after reversal of uremia.
Introduction
Cardiovascular disease (CVD) is highly prevalent and a leading cause of death among patients with chronic kidney disease (CKD).1 Left ventricular hypertrophy and systolic and diastolic dysfunction are well-recognized indicators of worse cardiovascular outcomes in patients undergoing dialysis. In advanced CKD, the myocardium is exposed to complex metabolic stressors that result from uremia-related inflammation, oxidative stress, renin-angiotensin-aldosterone system activation, calcitriol and klotho deficiency, increased fibroblast growth factor (FGF) 23, and changes in mineral metabolism.2 This exposure leads to myocyte hypertrophy, reduced myocardial capillarization, and nonvascularized interstitial fibrosis as well as arteriosclerosis and arterial stiffening.3,4 Together, these ultrastructural changes reduce pump efficiency and increase cardiac energy expenditure and myocardial oxygen consumption.
Kidney transplant is the optimal treatment for end-stage renal disease (ESRD) and is associated with reduced cardiovascular morbidity and improved quality of life and survival.5,6,7 Some echocardiographic studies8,9,10 have reported reduced left ventricular mass and improved left ventricular ejection fraction (LVEF), but these findings have been inconsistent. In fact, serial cardiac magnetic resonance imaging has failed to identify significant regression in left ventricular mass after transplant.11 However, imaging cardiac structure at rest rather than function under strain may be insufficiently sensitive to detect ultrastructural changes that occur with uremia or its reversal after transplant and may therefore fail to accurately reflect the risk of premature cardiovascular death among patients with CKD. Furthermore, pretransplant assessment and many trials in nephrology have traditionally relied on static measures derived from echocardiography or cardiac magnetic resonance imaging to identify risk and assess cardiovascular improvement or decline. To date, these indexes fail to accurately reflect the significant risk of premature cardiovascular death in the population with CKD and the overall functional capacity of this population.
Cardiovascular functional capacity or performance can be objectively assessed through quantification of cardiovascular reserve using cardiopulmonary exercise testing (CPET),12 which incorporates ventilatory gas exchange measurements during graded exercise. Maximum oxygen consumption (V̇O2max) and oxygen consumption at the point of anaerobic threshold (V̇O2AT) are objective, reproducible measures of cardiovascular reserve. These CPET-derived indexes reflect ventricular function (pumping capacity), vascular function (oxygen delivery), and skeletal muscle metabolic capacity (oxygen use).13,14,15 Moreover, they appear to be robust indicators of cardiovascular morbidity and premature death among patients with advanced CKD independent of left ventricular measures.7,16 Despite this, the use of CPET in nephrology is rare.
We hypothesized that kidney transplant is significantly associated with improvement in cardiovascular functional reserve. We conducted the prospective Cardiopulmonary Exercise Testing in Renal Failure and After Kidney Transplantation (CAPER) study to characterize changes in cardiovascular reserve using state-of-the-art CPET technology before and after kidney transplant in patients with ESRD who underwent kidney transplant compared with control individuals with ESRD who have not undergone kidney transplant and controls with hypertension and preserved kidney function. By assessing structural (echocardiography) and functional (CPET) alterations in parallel, CAPER is the first study, to our knowledge, to provide an integrated time-course assessment of critical prognostic variables that could allow robust cardiovascular risk stratification in the CKD population and inform future interventional trials.
Methods
Study Design
We conducted a 3-arm, prospective nonrandomized, controlled cohort study of patients with ESRD who underwent kidney transplant (KTR group), wait-listed patients with ESRD who did not undergo kidney transplant (NTWC group), and controls with hypertension but without CKD, CVD (heart failure, ischemic heart disease, or cerebrovascular disease), or diabetes (HTC group). Baseline data were collected from April 1, 2010, to January 1, 2013. Patients were followed up longitudinally for up to 1 year after kidney transplant. Clinical data collection was completed February 2014. Data analysis was performed from June 1, 2014, to March 5, 2015. Further analysis on baseline and prospective data was performed from June 1, 2017, to July 31, 2019. All data were deidentified. The study was approved by the Black Country Research Ethics Committee and adhered to the Declaration of Helsinki.17 All eligible participants provided written informed consent.
Patients were assessed at baseline, 2 months, and 1 year. Patients with ESRD who were aged 18 years or older and undergoing kidney transplant at the University Hospital Coventry and Warwickshire National Health Service Trust, Coventry, United Kingdom, were enrolled within the 4 weeks preceding transplant (eFigure 1 in the Supplement). Patients were included in the HTC group because of the near-ubiquitous presence of hypertension in patients with advanced CKD.12 Patients in the HTC group were recruited through a primary care database. Patients with preexisting chronic lung disease were excluded.
CPET and Echocardiography Assessment
All participants underwent CPET and echocardiography at baseline and after 2 and 12 months. For dialysis-dependent participants, all assessments were performed on a nondialysis day at least 12 hours after the last dialysis session.18,19 Patients in the HTC group were excluded from the 2-month assessment. Both CPET and echocardiography were performed as previously described,12 and protocol details are provided in the eMethods in the Supplement.
Study Outcomes
The primary study outcome was change in V̇O2max and V̇O2AT at 2 and 12 months from baseline in patients in the KTR vs NTWC groups. Secondary outcomes included change in V̇O2max and V̇O2AT at 12 months from baseline in patients in the KTR vs NTWC and HTC groups. A tertiary objective was to assess the correlation of changes in determinants of V̇O2max with change in V̇O2max from baseline to 2 and 12 months in each group.
Statistical Analysis
Continuous variables were summarized by using means (SDs) when normally distributed and by medians (interquartile ranges [IQRs]) otherwise. Categorical variables are presented as frequencies with percentages. Two group comparisons between patients in the KTR and NTWC groups were conducted by independent group t tests, Kruskal-Wallis test, or χ2 test, depending on variable type and distribution. Similarly, patients in the KTR, NTWC, and HTC groups were compared using 1-way analysis of variance, Kruskal-Wallis test, or χ2 tests as appropriate. Two-way repeated-measures analysis of variance with a time × group interaction term adjusted for covariates stated in the respective analysis presentation were fitted to assess the change over time and for the comparison of groups at follow-up time points. P values are presented for the within-patient × between-patient interaction using the Huynh-Feldt-Lecoutre correction. The nonparametric Friedman test was used for repeated-measures analysis when the assumptions for repeated-measures analysis of variance were not met. The statistical analysis was conducted in SAS software, version 9.4 TS1M2 (SAS Institute Inc), and P < .05 was regarded as statistically significant.
Results
Study Participants
On the 253 study participants (mean [SD] age, 48.5 [12.7] years; 141 [55.7%] male), 81 were in the KTR group, 85 in the NTWC group, and 87 in the HTC group. In the KTR group, 74 patients (91.4%) obtained a living donor kidney transplant. Maintenance immunosuppression after transplant consisted of combination treatment with corticosteroids (79 [97,5%]), tacrolimus (78 [96.3%]) or cyclosporine (1 [1.2%]), and azathioprine (41 [50.6%]) or mycophenolate mofetil (36 [44.4%]). Two-month assessments were completed by 73 patients (90.1%) in the KTR group and 81 (95.3%) in the NTWC group. Twelve-month assessments were completed by 68 patients (84.0%) in the KTR group, 61 (71.8%) in the NTWC group, and 71 (81.6%) in the HTC group (eFigure 1 in the Supplement). Of the 81 patients who underwent kidney transplant, no patients had serious infectious complications that required exclusion from the study. During the 12-month study period, 27 individuals required treatment for acute graft rejection episodes, but this did not preclude them from the study. The mean (SD) estimated glomerular filtration rate (eGFR) of all patients undergoing transplant was 55.3 (17.0) mL/min/1.73 m2 at 2 months and 59.1 (18.4) mL/min/1.73 m2 at 12 months (eTable 1 in the Supplement).
The mean (SD) age of patients in the KTR group was 43.1 (14.2) years compared with 49.7 (12.8) years in the NTWC group (P = .002) and 53.6 (8.0) years in the HTC group (P < .001). The mean (SD) body mass index (calculated as weight in kilograms divided by height in meters squared) was significantly lower in the KTR group (24.9 [3.8]) compared with the NTWC (26.9 [5.1]) and HTC (27.7 [3.4]) groups (P = .001 for both) (Table 1). Sex, race/ethnicity, prevalence of hypertension, duration of antihypertensive use, and tobacco smoking were not significantly different among the 3 groups. No significant differences were found in the antihypertensives used, diabetes or CVD prevalence, dialysis vintage, or hemoglobin, highly sensitive C-reactive protein, serum calcium, and albumin levels in the CKD groups.
Table 1. Baseline Characteristics of the Study Populationa.
| Characteristic | Advanced CKD | HTC Group (n = 87) | P Valueb | |
|---|---|---|---|---|
| KTR Group (n = 81) | NTWC Group (n = 85) | |||
| Male | 46 (56.8) | 53 (62.4) | 42 (48.3) | .12 |
| Race/ethnicity | ||||
| White | 66 (81.5) | 66 (77.7) | 81 (93.1) | .37 |
| Asian | 9 (11.1) | 13 (15.3) | 6 (6.9) | |
| Black | 6 (7.41) | 6 (7.1) | 0 (0) | |
| Age, mean (SD), y | 43.1 (14.2) | 49.7 (12.8) | 53.6 (8.0) | <.001 |
| BMI, mean (SD) | 24.9 (3.8) | 26.9 (5.1) | 27.7 (3.4) | .001 |
| SBP, mean (SD), mm Hg | 136.2 (20.2) | 133.1 (20.8) | 141.5 (13.2) | .01 |
| DBP, mean (SD), mm Hg | 81.8 (11.7) | 78.7 (12.3) | 85.7 (9.9) | <.001 |
| Mean arterial pressure, mean (SD), mm Hg | 100.0 (12.9) | 96.9 (13.8) | 104.3 (9.5) | <.001 |
| Hypertension | 73 (90.1) | 78 (91.8) | 87 (100) | .72 |
| Antihypertensive treatment duration, median (IQR), mo | 84 (36-180) | 102 (36-198) | 60 (36-120) | .14 |
| Blood pressure medication use | ||||
| ACEI or ARB blocker | 35 (43.2) | 33 (38.8) | 51 (58.6) | <.001 |
| Calcium antagonist | 42 (51.8) | 45 (52.9) | 39 (44.8) | |
| β-Blocker | 30 (37.0) | 33 (38.8) | 12 (13.8) | |
| Diuretic | 14 (17.3) | 13 (15.3) | 37 (42.5) | |
| Smoking (ever) | 39 (48.1) | 44 (51.8) | 46 (52.9) | .31 |
| Diabetes | 8 (9.9) | 11 (12.9) | 0 (0) | NA |
| Cardiovascular disease | 8 (9.9) | 9(10.6) | 0 (0) | NA |
| Dialysis status | ||||
| Predialysis | 28 (34.6) | 16 (18.8) | NA | NA |
| Hemodialysis | 45 (55.6) | 58 (68.2) | NA | NA |
| Peritoneal dialysis | 8 (9.9) | 11 (12.9) | NA | NA |
| Dialysis duration, mean (SD), mo | 33.2 (64.3) | 40.3 (44.6) | NA | NA |
| Transplant | ||||
| Living donor | 74 (91.4) | NA | NA | NA |
| Deceased donor | 7 (8.7) | NA | NA | NA |
| Laboratory values | ||||
| Creatinine level, mg/dL | 6.37 (5.20-8.79) | 7.71 (5.01-9.62) | 0.80 (0.69-0.95) | <.001 |
| eGFR, mL/min/1.73 m2 | 9.6 (4.1) | 8.9 (4.8) | 92.5 (15.0) | <.001 |
| Albumin level, g/dL | 4.4 (4.1-4.5) | 4.3 (4.1-4.5) | 4.7 (4.5-4.8) | <.001 |
| Corrected calcium level, mg/dL | 8.8 (0.8) | 9.2 (0.8) | 8.8 (0.4) | .47 |
| Phosphorus level, mg/dL | 4.95 (4.15-5.91) | 4.43 (3.72-5.08) | 3.28 (2.97-3.78) | <.001 |
| iPTH level, pg/mLb | 3.0 (1.1) | 3.0 (1.2) | 1.2 (0.4) | <.001 |
| hsCRP level, mg/Lb | 0.9 (1.3) | 1.2 (1.3) | 0.4 (1.0) | <.001 |
| Hemoglobin level, g/dL | 11.6 (10.9-12.7) | 11.9 (11.0-12.5) | 14.2 (13.3-14.9) | <.001 |
| HbA1c level, % | 5.5 (0.9) | 5.7 (1.0) | 5.7 (0.4) | .13 |
Abbreviations: ACEI, angiotensin-converting-enzyme inhibitor; ARB, angiotensin-receptor blocker; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CKD, chronic kidney disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; hsCRP, highly sensitive C-reactive protein; HTC, hypertension control; KTR, chronic kidney disease with kidney transplant; iPTH, intact parathyroid hormone; IQR, interquartile range; NA, not applicable; NTWC, wait-listed with chronic kidney disease without transplant; SBP, systolic blood pressure.
SI conversion factors: To convert albumin to grams per liter, multiply by 10; to convert calcium to millimoles per liter, multiply by 0.25; to convert creatinine to micromoles per liter, multiply by 88.4; to convert HbA1c to the proportion of hemoglobin, multiply by 0.01; to convert hemoglobin to grams per liter, multiply by 10; to convert hsCRP to nanomoles per liter, multiply by 9.524; to convert iPTH to nanograms per liter, multiply by 0.1053; and to convert phosphorus to millimoles per liter, multiply by 0.323.
Data are presented as number (percentage) of patients unless otherwise indicated.
Log transformed before analysis. The P value is for the 3-group analysis of variance comparison, Kruskal-Wallis test, or χ2 test (categorical variables).
Baseline Cardiovascular Functional and Structural Factors
All participants underwent CPET and performed maximal exercise that was accompanied by a median respiratory exchange ratio (ratio of carbon dioxide production to oxygen consumption) of 1.2 or greater (eTable 2 in the Supplement). The weight-standardized mean (SD) V̇O2max was lower in patients with advanced CKD (KTR group, 20.7 [5.8] mL · min−1 · kg−1; NTWC group, 18.9 [4.7] mL · min−1 · kg−1; P = .03) compared with the HTC group (24.9 [7.1] mL · min−1 · kg−1; P < .001). Mean (SD) V̇O2AT was lower in patients with advanced CKD (KTR group, 11.8 [2.3] mL · min−1 · kg−1; NTWC group, 11.4 [2.3] mL · min−1 · kg−1) compared with the HTC group (14.8 [3.8] mL · min−1 · kg−1; P < .001). Compared with those in the HTC group, patients with CKD had lower mean (SD) maximal workload (KTR group, 115.3 [50.7] W; NTWC group, 105 [36.7] W; HTC group, 156.4 [61.8] W; P < .001), median endurance time (KTR group, 10.3 [IQR, 9.0-11.7] min; NTWC group, 10.3 [IQR, 8.9-11.7] min; HTC group, 11.7 [IQR, 10.5-12.8] min; P < .001), and mean (SD) maximum heart rate (KTR group, 139.0 [22.9] min−1; NTWC group, 132.1 [18.5] min−1; HTC group, 155.1 [18.5] min−1; P < .001). The KTR and NTWC groups differed in baseline V̇O2max but not V̇O2AT, maximal workload, or endurance time.
Patients with CKD had higher mean (SD) left ventricular mass index (LVMI) (KTR group, 104.9 [36.1] g/m2; NTWC group, 113.8 [37.7] g/m2; HTC group, 87.8 [16.9] g/m2; P < .001), left ventricular end-diastolic volume index (KTR group, 48.2 [16.7] mL/m2; NTWC group, 51.0 [17.7] mL/m2; HTC group, 44.4 [10.2] mL/m2; P = .02), and left ventricular end-systolic volume index (KTR group, 19.8 [8.6] mL/m2; NTWC group, 20.6 [10.9] mL/m2; HTC group, 14.9 [4.6] mL/m2; P < .001) (eTable 2 in the Supplement). Mean (SD) LVEF was reduced in patients with CKD (KTR group, 60.1% [8.6%]; NTWC group, 61.4% [8.9%]) compared with the HTC group (66.1% [5.9%]) (P < .001), but other diastolic indexes were not significantly different among the 3 groups.
During the 12-month study period, a total of 13 participants in the KTR group, 24 in the NTWC group, and 16 in the HTC group were lost to follow-up (eFigure 1 in the Supplement). The major cardiovascular measures of V̇O2max, V̇O2AT, LVEF, LVMI, and E/mean e' at baseline between the 2 renal groups were not statistically different (eTable 3 in the Supplement).
Cardiovascular Functional and Structural Changes After Kidney Transplant
Transplant was associated with a restoration of eGFR in patients in the KTR group at 2 months (55.3 [17.0] mL/min/1.73 m2) and 12 months (59.1 [18.4] mL/min/1.73 m2) compared with the NTWC group (8.9 [4.8] mL/min/1.73 m2 at baseline, 9.2 [5.0] mL/min/1.73 m2 at 2 months, and 9.1 [4.3] mL/min/1.73 m2 at 12 months; P < .001). The mean (SD) phosphorus concentration decreased in the KTR group from baseline (5.26 [1.55] mg/dL) to 2 months (2.79 [0.62] mg/dL) and 12 months (2.79 [0.62] mg/dL) compared with the NTWC group (baseline: 4.64 [1.24] mg/dL; 2 months: 4.64 [1.24] mg/dL; 12 months:4.64 [1.24] mg/dL; P < .001). The mean (SD) intact parathyroid hormone level decreased in the KTR group from baseline (3.0 [1.1] pmol/L) to 2 months (2.1 [0.6] pmol/L) and 12 months (2.0 [0.6] pmol/L) compared with the NTWC group (baseline: 3.0 [1.2]; 2 months: 3.1 [1.2]; 12 months: 3.1 [1.0] pmol/L; P < .001) (to convert to nanograms per liter, multiply by 0.1053).
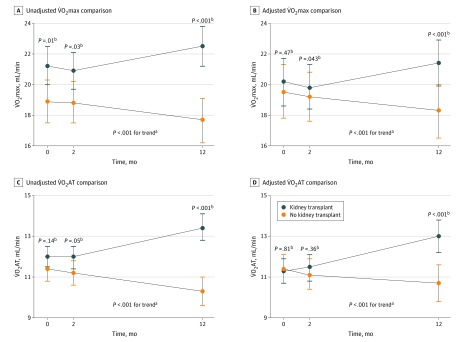
The mean (SD) V̇O2max increased at 12 months in patients in the KTR group (22.5 [6.3] mL · min−1 · kg−1; baseline: 20.7 [5.8] mL · min−1 · kg−1; P < .001) and compared with patients in the NTWC group (17.7 [4.1] mL · min−1 · kg−11; P < .001) in unadjusted models (Table 2 and Figure 1). The mean (SD) V̇O2AT improved at 12 months after transplant in the KTR group (13.4 [3.0] min/mL; baseline: 11.8 [2.3] mL · min−1 · kg−1; P < .001) vs the NTWC group (10.3 [2.0] mL · min−1 · kg−1; P < .001).
Table 2. Cardiovascular Measures at 2-Month and 12-Month Follow-up (Unadjusted).
| Measure | Time Point | P Valuea | ||
|---|---|---|---|---|
| Baseline | Month 2 | Month 12 | ||
| Functional Cardiovascular Reserve | ||||
| V̇O2max, mean (SD), mL · min−1 · kg−1 | ||||
| Transplant | 20.7 (5.8) | 20.9 (5.3) | 22.5 (6.3) | <.001 |
| Nontransplant | 18.9 (4.7) | 18.8 (4.6) | 17.7 (4.1) | |
| Control | 24.9 (7.1) | NA | 26.0 (7.1) | NA |
| P valueb | .01 | .03 | <.001 | NA |
| V̇O2AT, mean (SD), mL · min−1 · kg−1 | ||||
| Transplant | 11.8 (2.3) | 12.0 (2.3) | 13.4 (3.0) | <.001 |
| Nontransplant | 11.4 (2.3) | 11.2 (2.2) | 10.3 (2.0) | |
| Control | 14.8 (3.8) | NA | 15.0 (3.6) | NA |
| P valueb | .14 | .05 | <.001 | NA |
| Oxygen pulse, mean (SD), mL min−1 | ||||
| Transplant | 10.1 (8.4-12.9) | 10.4 (8.1-13.2) | 12.0 (9.9-14.8) | <.001 |
| Nontransplant | 11.0 (8.8-13.5) | 10.7 (8.7-13.3) | 11.5 (9.3-12.8) | |
| Control | 11.7 (9.4-14.7) | NA | 12.7 (10.0-16.4) | NA |
| P valueb | .61 | .47 | .09 | NA |
| Maximum workload, mean (SD), W | ||||
| Transplant | 115.3 (50.7) | 121.9 (51.1) | 133.3 (56.1) | <.001 |
| Nontransplant | 107.2 (36.7) | 108.7 (35.4) | 103.6 (31.3) | |
| Control | 156.4 (61.8) | NA | 158.0 (64.7) | NA |
| P valueb | .14 | .10 | <.001 | NA |
| Endurance time, median (IQR), min | ||||
| Transplant | 10.3 (9.0-11.7) | 10.5 (9.1-11.6) | 11.2 (9.6-12.7) | <.001 |
| Nontransplant | 10.3 (8.9-11.7) | 10.4 (9.3-11.8) | 9.8 (8.8-11.2) | |
| Control | 11.7 (10.5-12.8) | NA | 11.4 (10.1-13.0) | NA |
| P valueb | .91 | .85 | .002 | NA |
| Echocardiography Measures | ||||
| LVMI, mean (SD), g/m2 | ||||
| Transplant | 104.9 (36.1) | 115.1 (33.7) | 103.3 (26.8) | .01 |
| Nontransplant | 113.8 (37.7) | 110.5 (32.9) | 111.9 (35.4) | |
| Control | 87.8 (16.9) | NA | 85.4 (15.9) | NA |
| P valueb | .48 | .45 | .13 | NA |
| LVEF, mean (SD), % | ||||
| Transplant | 60.0 (8.6) | 62.2 (8.2) | 63.2 (6.8) | .02 |
| Nontransplant | 61.4 (8.9) | 61.3 (9.8) | 59.3 (7.6) | |
| Control | 66.1 (5.9) | NA | 66.0 (10.3 | NA |
| P valueb | .47 | .58 | .003 | NA |
| E/mean e', median (IQR) | ||||
| Transplant | 7.5 (6.0-9.6) | 8.0 (6.2-10.5) | 8.8 (6.7-11.2) | .49 |
| Nontransplant | 8.4 (6.8-10.4) | 8.6 (6.8-12.0) | 9.4 (7.2-11.7) | |
| Control | 8.3 (7.0-9.2) | NA | 8.2 (7.1-10.2) | NA |
| P valueb | .34 | .78 | .25 | NA |
| LA volume index, median (IQR), mL/m2 | ||||
| Transplant | 25.8 (19.1-30.5) | 27.9 (20.9-33.6) | 27.7 (20.3-34.9) | .27 |
| Nontransplant | 25.9 (19.0-37.1) | 28.0 (20.0-33.4) | 30.4 (21.8-39.3) | |
| Control | 25.4 (19.9-28.7) | NA | 26.8 (21.4-31.5) | NA |
| P valueb | .68 | .58 | .40 | NA |
Abbreviations: IQR, interquartile range; LA, left arterial; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; NA, not applicable; V̇O2AT, oxygen consumption at the point of anaerobic threshold; V̇O2max, maximum oxygen consumption.
Comparison of (time × group) interaction between the 2 renal groups using repeated-measure analysis of variance.
Comparison between all available groups at each respective time point.
Figure 1. Changes in Functional Cardiovascular Measures Before and After Kidney Transplant (Adjusted) .
Changes in maximum oxygen consumption (V̇O2max) and oxygen consumption at the point of anaerobic threshold (V̇O2AT) over time at baseline (before transplant), 2-month follow-up, and 12-month follow-up. Comparisons were adjusted for age, body mass index, sex, smoking, diabetes, cardiovascular disease, duration of antihypertensive therapy, β-blocker use, hemoglobin level, and dialysis duration.
aP value for comparison between 2 groups for changes over time.
bP value for comparison between 2 groups at each respective time point.
The CPET-derived variables were sensitive enough to detect reductions in cardiovascular functional reserve in patients in the NTWC group. An adjusted model (Figure 2) identified a decrease in cardiovascular function at 12 months in the NTWC group (median V̇O2max, −1.36 [IQR, −2.13 to −0.58] mL · min−1 · kg−1; median V̇O2AT: −1.02 [IQR, −1.34 to -0.70] mL · min−1 · kg−1)) and an improvement after 12 months in the KTR group (median V̇O2max, 0.99 [IQR, 0.22 to 1.77] mL · min−1 · kg−1; median V̇O2AT, 1.44 [IQR, 1.14 to 1.76] mL · min−1 · kg−1). However, cardiovascular functional reserve in patients in the KTR group did not return to levels observed in the HTC group (Figure 2).
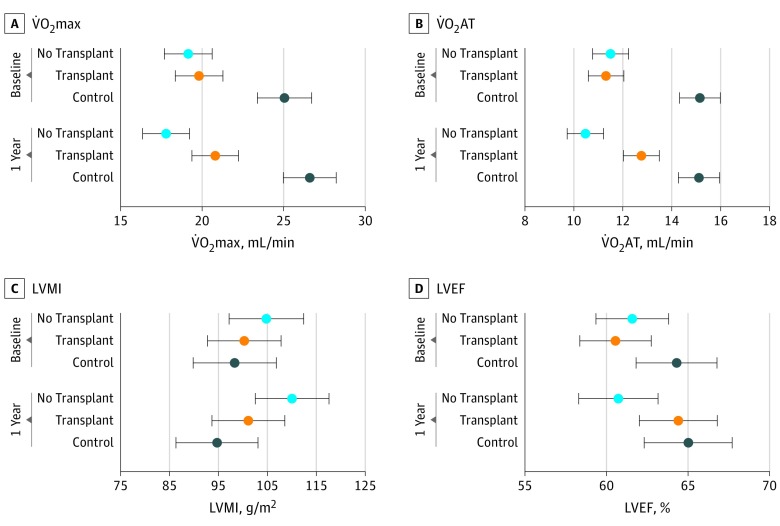
Figure 2. Changes in Functional and Structural Cardiovascular Measures Before and After Kidney Transplant.
Data are presented as means with 95% CIs (error bars) by group. Changes in maximum oxygen consumption (V̇O2max) and oxygen consumption at the point of anaerobic threshold (V̇O2AT), left ventricular mass index (LVMI), and left ventricular ejection fraction (LVEF) from baseline (before transplant) to 12-month follow-up, adjusted for age, body mass index, sex, smoking, diabetes, cardiovascular disease, duration of antihypertensive therapy, β-blocker use, hemoglobin level, and dialysis duration. P value for comparison among the 3 groups at 1-year follow-up with time × group interaction using analysis of variance.
The mean (SD) LVEF increased at 12 months in patients in the KTR group compared with baseline (63.2% [6.8%] vs 60.0% [8.6%], P = .02) and the NTWC group (59.3% [7.6%]) (P = .003) in all analyses (Table 2 and eFigure 2 in the Supplement), but transplant was not associated with improved LVMI at any time point in the adjusted model (eFigure 2 in the Supplement). Patients in the KTR group demonstrated increased mean (SD) maximal workload (KTR group, 133.3 [56.1] W; NTWC group, 103.6 [31.3] W; P < .001) and median endurance time (KTR group, 11.2 [IQR, 9.6-12.7] minutes; NTWC group, 9.8 [IQR, 8.8-11.2] minutes; P < .001) at 12 months.
Association of Cardiovascular Reserve With Kidney Transplant
In the KTR group, serum corrected calcium level (ρ = 0.30; 95% CI, 0.06-0.50; P = .01) and eGFR (ρ = 0.25; 95% CI, 0.00-0.46; P = .04) were significantly correlated with transplant-associated V̇O2max improvement (Table 3). Furthermore, the improvement in V̇O2max at 12 months after transplant was significantly correlated with the incremental 12-month change in the maximal workload (ρ = 0.66; 95% CI, 0.49-0.77; P < .001). In contrast to transplant recipients, hemoglobin level (ρ = 0.52; 95% CI, 0.31-0.68; P < .001) and LVEF (ρ = 0.31; 95% CI, 0.05-0.52; P = .02) were significantly correlated with V̇O2max, whereas LVMI (ρ = −0.37; 95% CI, −0.57 to −0.12; P = .004) had significant negative correlation with change in V̇O2max at the 12-month interval among the patients in the NTWC group.
Table 3. Correlation of Changes in Determinants of V̇O2max and Change in V̇O2max From Baseline to 12 Months for Each of the 3 Groups.
| Variable | Transplant | Nontransplant | Control | |||
|---|---|---|---|---|---|---|
| ρ (95% CI)a | P Value | ρ (95% CI)a | P Value | ρ (95% CI)a | P Value | |
| eGFR | 0.25 (0.00 to 0.46) | .04 | 0.22 (–0.03 to 0.45) | .09 | 0.05 (–0.19 to 0.29) | .67 |
| Corrected calcium level | 0.30 (0.06 to 0.50) | .01 | –0.02 (–0.27 to 0.24) | .90 | 0.08 (–0.16 to 0.32) | .49 |
| Phosphate level | 0.03 (–0.21 to 0.27) | .78 | –0.05 (–0.30 to 0.21) | .70 | 0.01 (–0.23 to 0.25) | .93 |
| iPTH levelb | –0.08 (–0.32 to 0.16) | .51 | 0.02 (–0.23 to 0.27) | .87 | –0.07 (–0.31 to 0.17) | .55 |
| hsCRP levelb | 0.18 (–0.08 to 0.42) | .17 | –0.07 (–0.32 to 0.19) | .59 | –0.08 (–0.32 to 0.16) | .51 |
| Hemoglobin level | 0.24 (0.00 to 0.45) | .05 | 0.52 (0.31 to 0.68) | <.001 | 0.31 (0.08 to 0.51) | .01 |
| LVMI | –0.10 (–0.33 to 0.15) | .44 | –0.37 (–0.57 to –0.12) | .004 | 0.23 (–0.01 to 0.45) | .06 |
| LVEF | –0.12 (–0.35 to 0.12) | .32 | 0.31 (0.05 to 0.52) | .02 | 0.06 (–0.18 to 0.30) | .61 |
| Mean arterial pressure | –0.04 (–0.28 to 0.20) | .74 | 0.11 (–0.15 to 0.35) | .42 | –0.11 (–0.34 to 0.13) | .38 |
| Maximum HR | 0.37 (0.14 to 0.56) | .002 | 0.34 (0.09 to 0.55) | .009 | –0.06 (–0.30 to 0.18) | .62 |
| Maximum HR, % predicted | 0.36 (0.13 to 0.55) | .003 | 0.36 (0.11 to 0.56) | .005 | –0.09 (–0.32 to 0.15) | .45 |
| Maximal workload | 0.66 (0.49 to 0.77) | <.001 | 0.46 (0.23 to 0.64) | <.001 | 0.71 (0.57 to 0.81) | <.001 |
Abbreviations: eGFR, estimated glomerular filtration rate; HR, heart rate; hsCRP, highly sensitive C-reactive protein; iPTH, intact parathyroid hormone; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; V̇O2max, maximum oxygen consumption.
The 95% CIs for the Pearson product-moment correlation coefficient (ρ) were derived using Fisher z transformation.
Log transformed before analysis.
At 2 months, systolic blood pressure and mean arterial pressure were significantly higher in the KTR group than in the NTWC cohort (eTable 4 in the Supplement). However, these blood pressure changes were not significantly different between the KTR and NTWC groups by 12 months. Furthermore, changes in mean arterial pressure from baseline to 12 months did not correlate with the changes in V̇O2max in the 3 study groups (Table 3). The improvement in V̇O2max at 12 months after transplant was significantly correlated with the incremental 12-month change in maximum heart rate (ρ = 0.37, P = .002) and percentage of predicted maximum heart rate (ρ = 0.36, P = .003) (Table 3).
Discussion
This study is the first, to our knowledge, to provide an integrated assessment of cardiovascular functional and morphologic changes in the population with CKD before and after kidney transplant. Kidney transplant was associated with significant improvement in V̇O2max and V̇O2AT during 12 months after transplant. This finding occurred with some improvement in LVEF but without any significant change in LVMI. This improvement in cardiovascular reserve was more meaningful when contrasted with the inverse direction of change in patients with advanced CKD who did not undergo transplant. Unlike the limited prognostic value of single surrogate factors of most established clinicopathologic factors (such as age, hypertension, LVMI, and LVEF), measures of cardiovascular reserve have been shown to be independently associated with survival in patients with advanced CKD (before and after kidney transplant) and after adjusting for known comorbid factors.7
In our study, transplant-associated improvements over time in cardiovascular reserve indexes (V̇O2max and V̇O2AT), oxygen pulse (a measure of oxygen consumption per heart beat) at maximal exercise, tolerated workload, and endurance time were significant even after adjusting for age, body mass index, sex, smoking, diabetes, CVD, duration of antihypertensive therapy, β-blocker use, hemoglobin level, and length of time patients are receiving dialysis. Although improvements in uremia and fluid overload were already notable at 2 months after transplant, the improvement in V̇O2max was only evident 12 months after transplant but did not improve to that observed in the control group without CKD. This finding is consistent with the incomplete normalization of kidney function after transplant, with the mean (SD) 12-month eGFR (59.1 [18.4] mL/min/1.73 m3) still consistent with CKD stage 3. Taken together, these data suggest that it takes several months for reversal of cardiovascular molecular and ultrastructural alterations that may be partially associated with uremia, to reverse sufficiently to result in detectable cardiovascular functional improvement.
Several uncontrolled prospective studies have evaluated pre– and post–renal transplant echocardiographic left ventricular dimensions.8,10,20 Ferreira et al8 reported significant LVMI regression (but no change in LVEF) in 24 transplant recipients after 12 months. In contrast, Hüting10 reported no change in LVMI and significant increases in LVEF at a mean of 41 months after transplant among 24 patients. In 29 patients with a pretransplant LVEF less than 50%, Melchor et al20 reported increases in LVEF 1 and 12 months after kidney transplant. These studies are limited by sample size, the lack of controls not receiving transplants, and the absence of data on the timing of echocardiography with dialysis,10,20 given the potential for confounding by intravascular volume or dialysis-induced myocardial stunning.19 A more recent study11 using volume-independent cardiac magnetic resonance imaging failed to show significant changes in LVMI and LVEF after transplant. Having controlled for dialysis-related changes in volume and myocardial contractility, we also found no significant differences in the in LVMI and left ventricular diastolic indexes over time between patients with advanced CKD who did and did not receive transplants.
Despite unaltered LVMI, we found an improvement in LVEF in the transplant group at 12 months compared with the nontransplant group. Taken together, the marked changes in measures of functional cardiovascular reserve and the subtle difference in LVEF in the absence of other significant structural echocardiographic changes reported here suggest that the reduction in cardiovascular mortality associated with kidney transplant may be explained by improved cardiovascular functional reserve.5,7 In addition, these results provide evidence that CPET-derived measures of cardiovascular functional reserve are more sensitive than left ventricular geometric measures for detecting cardiovascular improvement or decline or for risk determination in patients with CKD with shifting GFRs. Although the pathophysiologic origin of CVD in patients with CKD is multifactorial, measures of cardiovascular reserve obtained under incremental exercise load reflect overall circulatory health and the ability to respond to physiologic and pathologic cardiocirculatory stresses.7,20
The observation that transplant was associated with improved V̇O2max and V̇O2AT is important because elucidation of the mechanism could implicate therapeutic targets for improving cardiovascular outcomes in CKD. Our finding of a correlation of eGFR with change in V̇O2max is not surprising because eGFR serves as a surrogate for uremia. However, the correlation between corrected calcium level and change in V̇O2max is intriguing because perturbations in bone mineral metabolism are pathognomonic of uremia, and it is biologically plausible that calcium, which has a fundamental role in cardiomyocyte contractility and relaxation,21 could influence myocardial function in uremia. Intracellular calcium in cardiomyocytes is regulated by sodium-calcium exchange. Emerging evidence suggests that sodium-calcium exchange function is impaired in the context of uremia.22 However, if plasma-corrected calcium played a central role in determining V̇O2max, it might be expected that improvements in V̇O2max would be associated with changes in corrected calcium level, but we did not observe significant changes in calcium level after transplant. Ionized calcium is a more reliable indicator of calcium metabolism but was not determined in our study. Further work is needed to examine the extent to which calcium or modulators of calcium metabolism are mechanistically important; relevant factors may include vitamin D metabolites,23 FGF-23, and klotho.24,25 This finding suggests that partial reversal of the blunted heart rate response to maximal exercise in advanced CKD12 at 12 months after transplant is associated with improvement in functional cardiovascular reserve.
Although direct measurement of skeletal muscle strength was not performed in this study, we used the measures of V̇O2AT and maximal workload as indicators of greater skeletal muscle oxidative capacity and thus function. A curvilinear association exists between blood lactate values during exercise and endurance exercise performance velocity, leading to the concept that the rate of aerobic metabolism during skeletal muscle function can be accurately described by lactate production as reflected by V̇O2AT.26 We found that both V̇O2AT and maximal workload improved after kidney transplant at 12 months, which suggests improved skeletal muscle function; however, additional studies are needed to assess this finding. A prior study27 found parallel improvements in the isometric quadriceps strength and exercise cardiovascular reserve without significant changes in resting echocardiographic cardiac morphologic findings after 10 weeks of exercise training among patients undergoing dialysis in a randomized, assessor-blinded, clinical study. These results reflect the complex and intricate association between cardiovascular and skeletal health.
In contrast to patients in the KTR group, the continued decrease in V̇O2max in patients in the NTWC group was significantly correlated with hemoglobin and LVEF and inversely correlated with LVMI. Other likely contributors include myocardial maladaptation through fibrosis, hypertrophy and capillary rarefaction known to occur in uremia, fluid shifts and myocardial stunning on dialysis, and circulating factors pathognomonic of uremia, including FGF-23, klotho, and others.
Strengths and Limitations
Our study has several strengths. It was considerably larger than previous studies10,20 that reported echocardiographic changes and, to our knowledge, was the first prospective, longitudinal cohort study to evaluate functional reserve using CPET, to standardize the timing of evaluations in relation to dialysis, to control for volume status and myocardial contractility, and to include control groups of individuals with hypertension but without CKD who did not receive transplants.
However, our findings should be interpreted against the limitations of our study. First, randomization was not possible in this setting, and there were significant baseline differences in known variables associated with CV risk, including age, weight, and blood pressure, although we adjusted for these factors in multivariate analyses. Second, cardiac magnetic resonance imaging may be a more sensitive method to assess structural cardiac changes compared with echocardiography, and it is possible that the use of echocardiography in our study may have underestimated posttransplant structural changes. Third, noninvasive measures of cardiac output were not assessed in this study, but evaluation of these during CPET in future studies may provide further insight into physiologic changes that occur before and after kidney transplant. A limitation of CPET technology is that it inherently selects for patients who retain some functional ability to exercise. However, this is partially circumvented by the assessment of submaximal indexes, such as V̇O2AT, that provide several advantages, including ease of ascertainment during low-level exercise, relevance to ability to perform activities of daily living, and independence from volitional exercise effort. In the present study, although no patients underwent reversal of arteriovenous fistula, arteriovenous fistula or its closure on cardiac volumes may influence CPET measurements, which would need to be assessed during future studies.
Conclusion
Our study found that partial restoration of kidney function by transplant was significantly associated with improved cardiovascular functional reserve as assessed by CPET, without major change in ventricular structural morphologic features. The CPET-derived indexes were also sensitive enough to detect a decrease in cardiovascular functional reserve in wait-listed patients with CKD who did not receive transplants. The study appears to provide insight on cardiovascular structural-functional dynamics and the association of kidney function restoration with cardiovascular physiologic findings. The data presented indicate that V̇O2max may be a sensitive index for assessing cardiovascular function and stratifying risk in patients with renal impairment.
eMethods. Supplemental Methods
eFigure 1. Number of Patients Enrolled and Completed Study
eFigure 2. Changes in Echocardiographic Measures Before and After Kidney Transplantation (Adjusted)
eTable 1. Laboratory Measures at Baseline, 2-Months, and 12-Months Follow-up (Unadjusted)
eTable 2. Baseline Cardiovascular Characteristics
eTable 3. Baseline Characteristics of Patients Lost to Follow-up During the 12-Month Study Period
eTable 4. Cardiovascular Functional and Hemodynamic Measures
References
- 1.Methven S, Steenkamp R, Fraser S. UK Renal Registry 19th annual report: chapter 5 survival and causes of death in UK adult patients on renal replacement therapy in 2015: national and centre-specific analyses. Nephron. 2017;137(suppl 1):117-150. doi: 10.1159/000481367 [DOI] [PubMed] [Google Scholar]
- 2.de Borst MH, Vervloet MG, ter Wee PM, Navis G. Cross talk between the renin-angiotensin-aldosterone system and vitamin D-FGF-23-klotho in chronic kidney disease. J Am Soc Nephrol. 2011;22(9):1603-1609. doi: 10.1681/ASN.2010121251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aoki J, Ikari Y, Nakajima H, et al. . Clinical and pathologic characteristics of dilated cardiomyopathy in hemodialysis patients. Kidney Int. 2005;67(1):333-340. doi: 10.1111/j.1523-1755.2005.00086.x [DOI] [PubMed] [Google Scholar]
- 4.Edwards NC, Moody WE, Chue CD, Ferro CJ, Townend JN, Steeds RP. Defining the natural history of uremic cardiomyopathy in chronic kidney disease: the role of cardiovascular magnetic resonance. JACC Cardiovasc Imaging. 2014;7(7):703-714. doi: 10.1016/j.jcmg.2013.09.025 [DOI] [PubMed] [Google Scholar]
- 5.Meier-Kriesche HU, Schold JD, Srinivas TR, Reed A, Kaplan B. Kidney transplantation halts cardiovascular disease progression in patients with end-stage renal disease. Am J Transplant. 2004;4(10):1662-1668. doi: 10.1111/j.1600-6143.2004.00573.x [DOI] [PubMed] [Google Scholar]
- 6.Patel RK, Mark PB, Johnston N, et al. . Prognostic value of cardiovascular screening in potential renal transplant recipients: a single-center prospective observational study. Am J Transplant. 2008;8(8):1673-1683. doi: 10.1111/j.1600-6143.2008.02281.x [DOI] [PubMed] [Google Scholar]
- 7.Ting SM, Iqbal H, Kanji H, et al. . Functional cardiovascular reserve predicts survival pre-kidney and post-kidney transplantation. J Am Soc Nephrol. 2014;25(1):187-195. doi: 10.1681/ASN.2013040348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferreira SR, Moisés VA, Tavares A, Pacheco-Silva A. Cardiovascular effects of successful renal transplantation: a 1-year sequential study of left ventricular morphology and function, and 24-hour blood pressure profile. Transplantation. 2002;74(11):1580-1587. doi: 10.1097/00007890-200212150-00016 [DOI] [PubMed] [Google Scholar]
- 9.Montanaro D, Gropuzzo M, Tulissi P, et al. . Effects of successful renal transplantation on left ventricular mass. Transplant Proc. 2005;37(6):2485-2487. doi: 10.1016/j.transproceed.2005.06.022 [DOI] [PubMed] [Google Scholar]
- 10.Hüting J. Course of left ventricular hypertrophy and function in end-stage renal disease after renal transplantation. Am J Cardiol. 1992;70(18):1481-1484. doi: 10.1016/0002-9149(92)90303-G [DOI] [PubMed] [Google Scholar]
- 11.Patel RK, Mark PB, Johnston N, McGregor E, Dargie HJ, Jardine AG. Renal transplantation is not associated with regression of left ventricular hypertrophy: a magnetic resonance study. Clin J Am Soc Nephrol. 2008;3(6):1807-1811. doi: 10.2215/CJN.01400308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ting SM, Hamborg T, McGregor G, et al. . Reduced cardiovascular reserve in chronic kidney failure: a matched cohort study. Am J Kidney Dis. 2015;66(2):274-284. doi: 10.1053/j.ajkd.2015.02.335 [DOI] [PubMed] [Google Scholar]
- 13.Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH Jr, Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83(3):778-786. doi: 10.1161/01.CIR.83.3.778 [DOI] [PubMed] [Google Scholar]
- 14.American Thoracic Society; American College of Chest Physicians . ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167(2):211-277. doi: 10.1164/rccm.167.2.211 [DOI] [PubMed] [Google Scholar]
- 15.Hunt SA; American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) . ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). J Am Coll Cardiol. 2005;46(6):e1-e82. doi: 10.1016/j.jacc.2005.08.022 [DOI] [PubMed] [Google Scholar]
- 16.Ting SM, Iqbal H, Hamborg T, et al. . Reduced functional measure of cardiovascular reserve predicts admission to critical care unit following kidney transplantation. PLoS One. 2013;8(5):e64335. doi: 10.1371/journal.pone.0064335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 18.Parfrey PS, Foley RN, Harnett JD, Kent GM, Murray D, Barre PE. Outcome and risk factors of ischemic heart disease in chronic uremia. Kidney Int. 1996;49(5):1428-1434. doi: 10.1038/ki.1996.201 [DOI] [PubMed] [Google Scholar]
- 19.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced repetitive myocardial injury results in global and segmental reduction in systolic cardiac function. Clin J Am Soc Nephrol. 2009;4(12):1925-1931. doi: 10.2215/CJN.04470709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melchor JL, Espinoza R, Gracida C. Kidney transplantation in patients with ventricular ejection fraction less than 50 percent: features and posttransplant outcome. Transplant Proc. 2002;34(7):2539-2540. doi: 10.1016/S0041-1345(02)03478-4 [DOI] [PubMed] [Google Scholar]
- 21.Hohendanner F, Ljubojević S, MacQuaide N, et al. . Intracellular dyssynchrony of diastolic cytosolic [Ca2+] decay in ventricular cardiomyocytes in cardiac remodeling and human heart failure. Circ Res. 2013;113(5):527-538. doi: 10.1161/CIRCRESAHA.113.300895 [DOI] [PubMed] [Google Scholar]
- 22.McMahon AC, Naqvi RU, Hurst MJ, Raine AE, MacLeod KT. Diastolic dysfunction and abnormality of the Na+/Ca2+ exchanger in single uremic cardiac myocytes. Kidney Int. 2006;69(5):846-851. doi: 10.1038/sj.ki.5000193 [DOI] [PubMed] [Google Scholar]
- 23.Choudhury S, Bae S, Ke Q, et al. . Abnormal calcium handling and exaggerated cardiac dysfunction in mice with defective vitamin D signaling. PLoS One. 2014;9(9):e108382. doi: 10.1371/journal.pone.0108382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim K, Lu TS, Molostvov G, et al. . Vascular klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation. 2012;125(18):2243-2255. doi: 10.1161/CIRCULATIONAHA.111.053405 [DOI] [PubMed] [Google Scholar]
- 25.Lim K, Groen A, Molostvov G, et al. . α-Klotho expression in human tissues. J Clin Endocrinol Metab. 2015;100(10):E1308-E1318. doi: 10.1210/jc.2015-1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joyner MJ, Coyle EF. Endurance exercise performance: the physiology of champions. J Physiol. 2008;586(1):35-44. doi: 10.1113/jphysiol.2007.143834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGregor G, Ennis S, Powell R, et al. . Feasibility and effects of intra-dialytic low-frequency electrical muscle stimulation and cycle training: a pilot randomized controlled trial. PLoS One. 2018;13(7):e0200354. doi: 10.1371/journal.pone.0200354 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Supplemental Methods
eFigure 1. Number of Patients Enrolled and Completed Study
eFigure 2. Changes in Echocardiographic Measures Before and After Kidney Transplantation (Adjusted)
eTable 1. Laboratory Measures at Baseline, 2-Months, and 12-Months Follow-up (Unadjusted)
eTable 2. Baseline Cardiovascular Characteristics
eTable 3. Baseline Characteristics of Patients Lost to Follow-up During the 12-Month Study Period
eTable 4. Cardiovascular Functional and Hemodynamic Measures