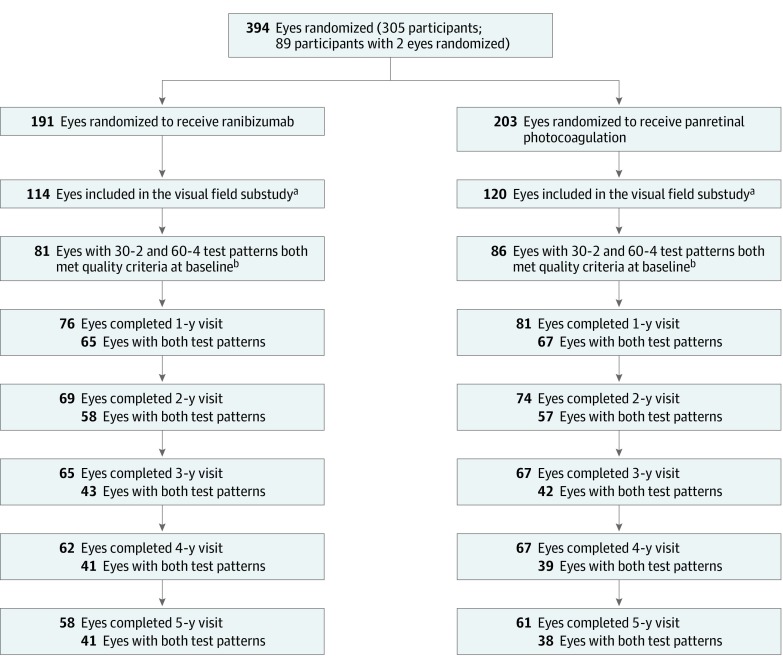
Figure 1. Flowchart of Eyes Included in the Ancillary Study on Visual Fields of Participants Enrolled in the Protocol S Clinical Trial.
aDefined as eyes with at least 1 test pattern (either Humphrey Field Analyzer [HFA] 30-2 or HFA 60-4) available at baseline.
bDefined as eyes with both test patterns (HFA 30-2 and HFA 60-4) available and excluding eyes that had excessive false positive or excessive fixation loss at baseline or other irregularities invalidating the results. For HFA 30-2 test, 28 and 32 scans were excluded from ranibizumab and panretinal photocoagulation groups, respectively; for HFA 60-4 test, 30 and 31 scans were excluded, respectively.