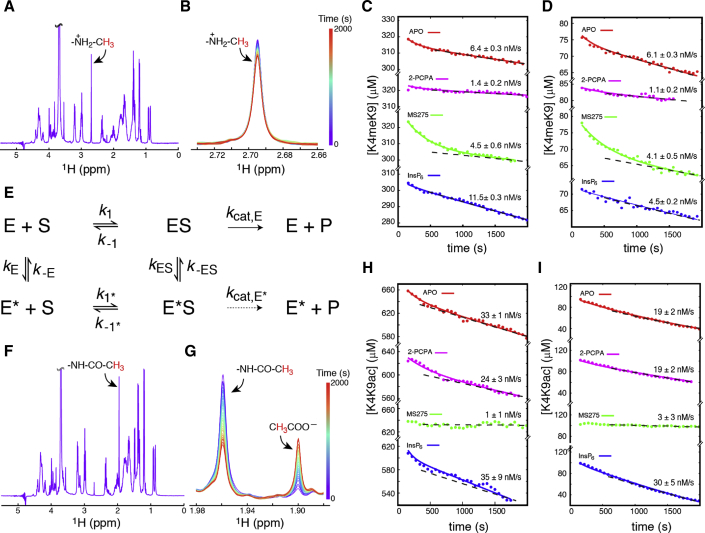
Figure 2.
Enzymatic Coupling between LSD1 and HDAC1 in the CoREST Complex
(A) The 1H NMR reference spectrum of a 330 μM sample of H3K4me, with the assignment of the K4 N(6) methyl protons shown.
(B) Time series after the addition of 200 nM CoREST complex to the H3K4me substrate.
(C and D) Progression curves for the conversion of ca. 300 μM (C) or 80 μM (D) H3K4me substrate incubated with 200 nM CoREST complex.
(E) Filled circles are experimentally obtained substrate concentrations versus time, full-drawn lines are the results of the least-squares fits to the reaction scheme, and dashed lines represent the limiting rates after equilibrium is reached.
(F) Reference 1H NMR spectrum of 670 μM H3K9ac with the assignment of the methyl protons of the K9 acetyl group shown.
(G) A representative time series obtained after the addition of 50 nM CoREST complex to a 100 μM sample of H3K9ac. It is noted that both the disappearance of the H3K9ac substrate and the appearance of the acetate product can be observed, and their concentrations can be quantified from the intensity of the two peaks.
(H and I) Progression curves for ca. 660 μM (H) or 100 μM (I) H3K9ac substrate concentration versus time after addition of 50 nM CoREST.