Figure 1.
Sup35 Peptide p103–113 Fibrils Accelerate Sup35 NM Aggregation In Vitro
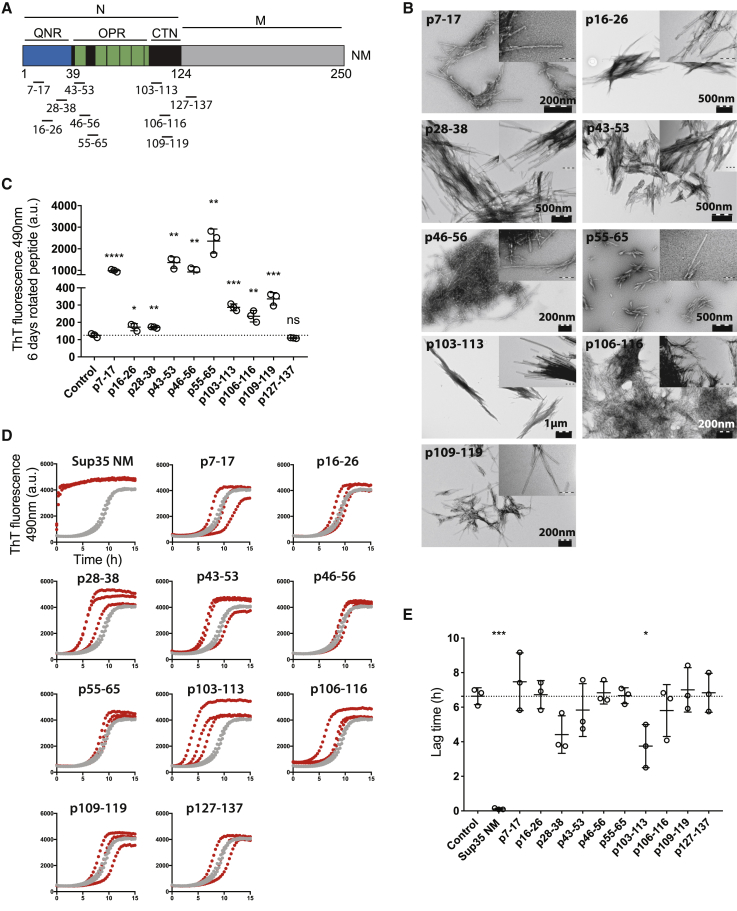
(A) Schematic diagram of functional domains N and M of Sup35. The regions corresponding to the synthetic peptides are marked. QNR, amino-terminal Q/N-rich region; OPR, oligopeptide repeat region; CTN carboxy-terminal N region.
(B) Synthetic peptides, rotated at 1 mM for 6 days, as observed by transmission electron microscopy (TEM). Indents represent higher magnification of the same image. p127–p137 aggregates were not observed by TEM and hence are not shown.
(C) ThT fluorescence of synthetic peptides rotated at 1 mM concentration for 6 days. Mean values and standard deviation (SD) of three independent replicates are shown. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.0001, and ∗∗∗∗p < 0.00001 (unpaired t test [every condition compared to vehicle control]).
(D) ThT fluorescence of recombinant Sup35 NM (10 μM) incubated without (gray) or with 2% (v/v) preformed, sonicated fibrils (red). Three independent replicates are shown.
(E) Mean lag time and SD of three independent repeats of the data in (D). ∗p < 0.05 and ∗∗∗p < 0.0001 (unpaired t test [every condition compared to vehicle control]).