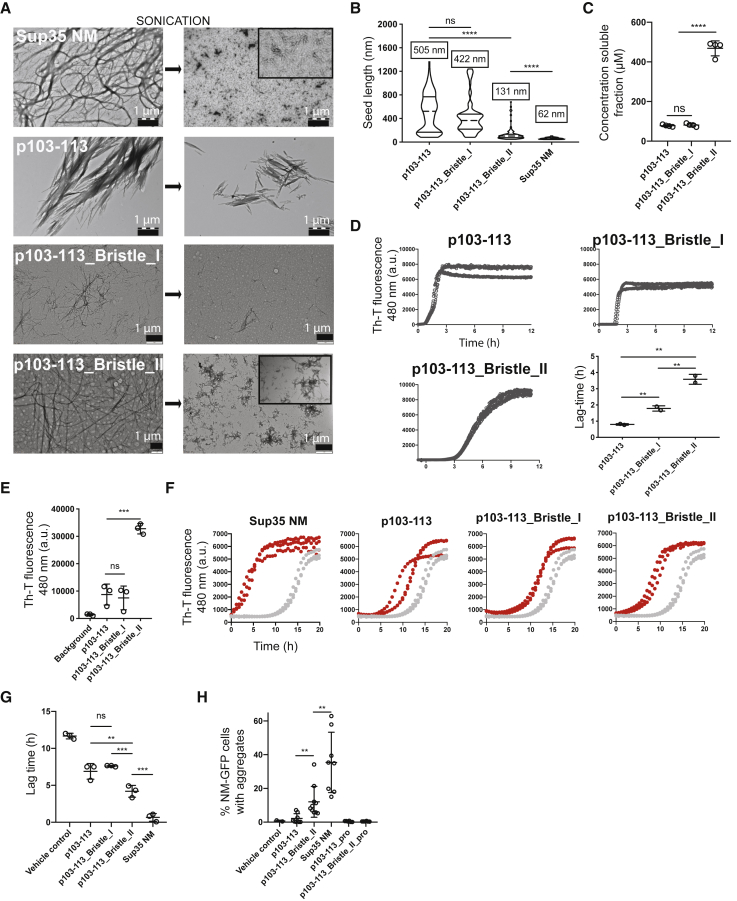
Figure 4.
Fibril Brittleness Increases Seeding Efficiency
(A) TEM images of preformed fibrils of Sup35 NM (10 μM monomer concentration), p103–113 (1 mM monomer concentration), p103–113_Bristle_I (1 mM monomer concentration), and p103–113_Bristle_II (1 mM monomer concentration) before and after sonication.
(B) Quantification of the length of individual aggregates (seeds) after sonication based on four independent TEM experiments, visualized as violin plots. Numbers above violin symbols represent average size of seeds. ∗∗∗∗p < 0.00001 (one-way ANOVA).
(C) Concentrations of the soluble fractions of peptides rotated for 6 days at 1 mM. ∗∗∗∗p < 0.00001 (one-way ANOVA).
(D) ThT kinetic assay of all peptides at 1 mM with continuous shaking. Three independent replicates are shown. Quantification of the lag time of each of the sigmoidal curves is shown on the bottom right. Mean values and SD of three independent replicates are shown. ∗∗p < 0.01 (one-way ANOVA).
(E) ThT fluorescence of synthetic peptides rotated at 1 mM concentration for 6 days. Mean values and SD of three independent replicates are shown. ∗∗∗p < 0.0001 (one-way ANOVA).
(F) ThT kinetic assay of recombinant Sup35 NM (10 μM) incubated without (gray) or with 2% (v/v) preformed, sonicated fibrils (red). Three independent replicates are shown.
(G) Mean lag time and SD of three independent repeats of the data in (F). ∗∗p < 0.01 and ∗∗∗p < 0.0001 (one-way ANOVA).
(H) Induction of NM-GFP aggregates in recipient N2a NM-GFPsol cells. 1 mM peptides were fibrillized for 6 days, sonicated, and subsequently added to NM-GFPsol cells at a final concentration of 20 μM (monomer concentration). Shown is the mean ± SD (n > 4). ∗∗p < 0.01.
See also Figures S3–S6.