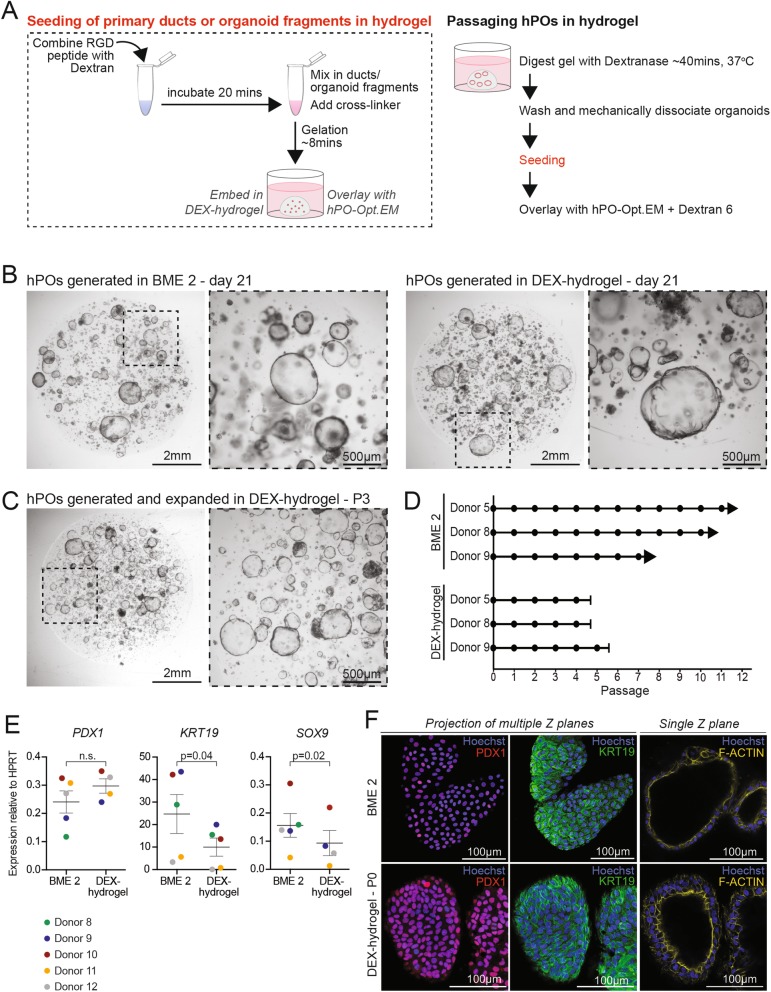
Fig. 5.
A chemically defined Dextran-based hydrogel supports hPO growth. Organoids were derived and expanded from freshly isolated human pancreas tissue either in standard BME 2 as ECM or in the chemically defined dextran-based hydrogel (DEX-hydrogel). a Schematic showing the workflow to use DEX-hydrogel as ECM when seeding ductal fragments for hPO culture initiation or organoid fragments during passaging (left panel). During passaging, dextranase is used to digest the hydrogel and Dextran 6 is added to the culture medium thereafter to prevent hydrogel breakdown (right panel), see methods for details. b Representative images of hPO cultures derived from freshly isolated human pancreas tissue and initiated in BME 2 (left) or DEX-hydrogel (right). Pictures were taken 21 days after seeding. c-d hPOs can be passaged up to passage 4 when cultured in DEX-hydrogel. Note that, hPOs in DEX-hydrogel expand to a lesser extent than those with BME 2 and cultures begin to deteriorate after P4. c Representative images of hPOs in DEX-hydrogel at P3 (n = 3). d Graph represents the expansion potential of independent donors cultured with BME 2 or DEX-hydrogel. (circle = passage, arrows indicate ongoing cultures, capped lines indicate cultures that deteriorated). e mRNA expression analysis of hPO cultures (P1-P4) reveals that organoids grown with DEX-hydrogel retain the expression of ductal and pancreatic genes although KRT19 and SOX9 are at a lower level than those cultured with BME 2 (Statistical analysis with paired t-test). f Immunofluorescence staining reveals normal cellular polarisation of hPOs in DEX-hydrogel and that the protein expression of ductal and pancreatic markers is maintained in DEX-hydrogel compared to BME 2 (F-Actin - yellow; PDX1 - red; KRT19 - green; Nuclei were counterstained with Hoechst - blue). Experiments were performed in n = 2 independent donors