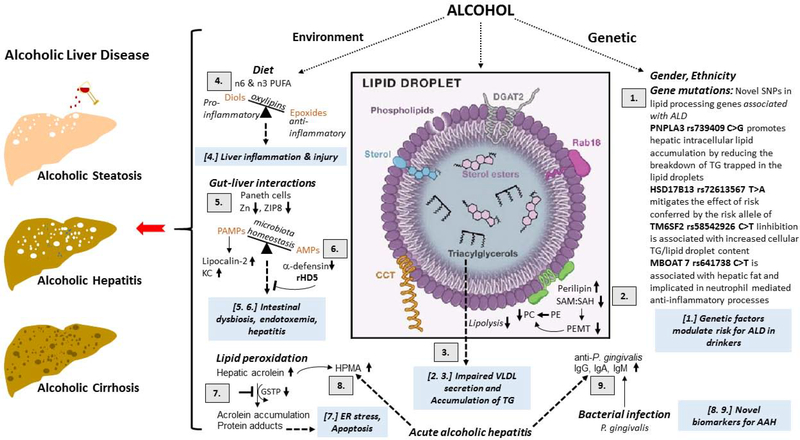
Figure 4.
Schematic of the update on the mechanistic and therapeutic viewpoint on the role of genetics and nutrition in the development of alcoholic liver disease (ALD).
[1.] GWAS identified SNPs in lipid processing pathways are associated with risk of progressing ALD. PNPLA3 rs739409 C>G promotes hepatic intracellular lipid accumulation by reducing the breakdown of TG trapped in the lipid droplets. HSD17B13 rs72613567 T>A mitigates the effect of risk conferred by the risk allele of PNPLA3. TM6SF2 rs58542926 C>T (E167K) inhibition is associated with increased cellular TG and lipid droplet content. MBOAT7 rs641738: C>T is associated with hepatic fat and implicated in neutrophil mediated anti-inflammatory processes.
[2.] Alcohol consumption decreases the phosphatidylethanolamine methyltransferase (PEMT)-catalyzed generation of the PC from PE that lowers the ratio of these two major classes of phospholipids on the lipid droplet (LD) surface monolayer. This change in the PC:PE ratio promotes the generation of enlarged LDs as well as recruits’ specific proteins such as perilipins 2 to the LD surface.
[3.] Supersized LDs and increase in specific perilipins prevents the access of lipases to LD triglyceride stores to inhibit their lipolysis thus facilitating lipid over-accumulation in the liver contributing to the development of alcoholic steatosis.
[4.] Ethanol-induced liver injury is associated with distinct profile of bioactive n6 and n3 PUFA metabolites. CYPs metabolize numerous PUFAs and generate a variety of different signalling oxidised lipids (oxylipins), including anti-inflammatory epoxy-FAs, which are rapidly hydrolyzed by sEH to their corresponding vicinal diols, dihydroxy-FAs, which are less active or might be pro-inflammatory. Possibly the deleterious effects of pro-inflammatory oxylipins outweigh the benefits of anti-inflammatory and pro-resolving lipid mediators and tip the balance toward liver damage.
[5.] Chronic alcohol impairs zinc transporter, ZIP8, activity leading to zinc deficiency in Paneth cells. The consequent reduction in antimicrobial peptides (AMPs, a-defensin) allows for pathogen associated molecular patterns (PAMPS) translocation from the intestine to the liver to induce pro-inflammatory lipocalin-2 and KC chemokine production, thereby contributing to alcoholic hepatitis.
[6.] Restoring a-defensin by recombinant human defensin 5 (rDH5) restores AMP, reduces intestinal dysbiosis, endotoxemia and hepatitis and is potentially a new class of therapeutic for treating alcoholic hepatitis.
[7.] Alcohol consumption and metabolism increase lipid peroxidation and generate acrolein. The concomitant alcohol-reduced reduction in glutathione-S-transferase Pi (GSTP) impairs the clearance of acrolein facilitating its accumulation and protein adduct formation causing ER stress.
[8.] Acrolein metabolite 3-hydroxypropyl-mercapturic acid (HMPA) is increased in urine of acute alcoholic hepatitis (AAH) patients. Urine HPMA may be a good non-invasive biomarker for severe AAH. These findings highlight the importance of lipids and lipid peroxidation products in AAH.
[9.] P. gingivalis is a major periodontal pathogen that can also affect remote organs such as the liver. Plasma levels of IgG, IgA and IgM against P. gingivalis strains are increased in severe acute alcoholic hepatitis and can serve as biomarkers for severity of AAH.