Abstract
Background
Glucocorticoids are important components of a number of chemotherapeutic regimens used to treat pediatric acute lymphoblastic leukemia (ALL). A primary cause of treatment failure of ALL is acquired resistance to glucocorticoids. Recently, traditional Chinese medicines were effectively used to treat solid tumors. Thus, the aim of this study was to investigate whether Huai Qi Huang (HQH), a traditional Chinese medicine, increased the efficacy of glucocorticoids in the treatment of ALL, and if so, to determine the underlying mechanism.
Material/Methods
Various concentrations of HQH were used to treat Jurkat and Nalm-6 cells for 24 to 72 hours. Subsequently, cells were co-treated with HQH and the glucocorticoid receptor agonist, dexamethasone (DEX), or a MEK inhibitor (PD98059) to verify the synergistic effects on apoptosis in Jurkat and Nalm-6 cells for 24 hours. Cell Counting Kit-8 assay and flow cytometry were used to measure cell viability and apoptosis, respectively. Protein and mRNA expression levels were assessed using western blotting and quantitative polymerase chain reaction.
Results
The results revealed that cell survival was reduced and apoptosis was increased as the HQH concentration was increased, and this was accompanied with increases in the levels of BAX, cleaved-caspase-3 and glucocorticoid receptor α (GRα) and decreases in the levels of Bcl-2 and phospho-ERK (pERK). Glucocorticoid receptor β (GRβ) and total ERK (t-ERK) had no significant changes. Combined treatment with HQH and DEX or PD98059 increased apoptosis in Jurkat and Nalm-6 cells, and concurrently increased BAX, cleaved-caspase-3, GILZ, NFKBIA, and GRα and decreased Bcl-2 and pERK.
Conclusions
HQH enhanced the sensitivity of ALL cells to glucocorticoids by increasing the expression of GRα and inhibiting the MEK/ERK pathway, thus providing a rational foundation for the treatment of ALL with HQH.
MeSH Keywords: Apoptosis; MAP Kinase Kinase Kinases; MAP Kinase Signaling System; Medicine, Chinese Traditional; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Receptors, Glucocorticoid
Background
Precursor B- or T-cell acute lymphoblastic leukemia (B-ALL or T-ALL) is a malignant hematopoietic disease that has different outcomes and prognosis. T-ALL accounts for 12% to 15% of new ALL cases in children and has a poorer prognosis than B-ALL [1]. The survival rates of pediatric patients with ALL has significantly improved in recent years, with advances in chemotherapy. However, overall, cancer causes the main death in children in the United States due to a relatively high rate of relapse (~20%) and a poor prognosis following relapse [2]. Glucocorticoids are important for successfully treating ALL because of their ability to promote differentiation and apoptosis [3]. Glucocorticoids rapidly reduce the burden on ALL tumor without suppressing the bone marrow. [4]. However, certain patients with ALL develop resistance to glucocorticoid agonists [5–7]. Therefore, there is an urgent requirement to develop novel drugs to reverse resistance or improve sensitivity to chemotherapy [8–10]. Plants are likely to be a source of anticancer drugs [11]. Different components in botanicals may not only have synergistic activities, but also buffer the toxic effects of therapeutics with a single constituent [12]. China has a rich history regarding the use of traditional Chinese medicines (TCMs) for various diseases and there are an increasing number of studies demonstrating their efficacy for treating patients with cancer. Huaier is the primary ingredient of Huang Qi Huai (HQH), which includes Trametes robiniophila Murr (Huaier), polygonatum, and Chinese wolfberry fruit. Huaier has been used in TCM for ~1600 years [13]. Studies have shown that Huaier extract exhibits anti-tumor effects in a number of solid tumors [14–22], and according to recent reports, HQH induces apoptosis in ALL cells [23]. However, whether HQH exhibits a synergistic effect with glucocorticoids when used to treat ALL remains unknown. Therefore, this study aimed to explore the effects of HQH as a potential treatment for ALL, whether HQH could accentuate the effects of glucocorticoids and determine the underlying mechanism.
Material and Methods
Cell lines and reagents
Jurkat and Nalm-6 cells were purchased from ATCC. HQH electuary was a kind gift from Qidong Gaitianli Medicine Co., Ltd. To prepare HQH for use, 1 g electuary ointment was dissolved in 1 mL 0.9% sodium chloride solution. The solution was filter sterilized with a 0.22 μm filter and stored at −20°C. Dexamethasone (DEX) was purchased from TCI Shanghai Development Co., Ltd. and PD98059 (cat. no. A1663) was obtained from APExBIO Technology LLC., and dissolved in ethanol or dimethyl sulfoxide (DMSO), respectively. Cell Counting Kit-8 (CCK-8) (Dojindo Molecular Technologies, Inc.) and Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis detection kit (BD Biosciences) were obtained to detect cell proliferation and apoptosis, respectively. To perform reverse transcription-quantitative polymerase chain reaction (RT-qPCR), RNAiso Plus, PrimeScript RT Master mix and TB Green Premix Ex Taq were purchased from Takara Bio Inc. Antibodies against ERK (cat. no. 4695T), phosphor-ERK (pERK; cat. no. 4370T), Bax (cat. no. 5023T), Bcl-2 (cat. no. 4223T), cleaved-caspase-3 (cleaved at Asp175; cat. no. 9579T), β-Actin (cat. no. 4970T), histone H3 (cat. no. 4499T) and horseradish peroxidase (HRP)-conjugated sheep anti-rabbit secondary antibody (cat. no. 7074P2) were all purchased from Cell Signaling Technology, Inc. Antibodies against GRα (cat. no. ab3580) and GRβ (cat. no. ab3581) were obtained from Abcam. Concentrated normal goat serum (Boster, AR1009) and goat anti-rabbit fluorescein (Cy3) conjugated secondary antibody (Boster, BA1032) were purchased from Wuhan, China. Triton X-100 (Beyotime, ST795) and 4,6-diamidino-2-phenylindole (DAPI) (Beyotime, C1002) were purchased from Shanghai, China. Enhanced chemiluminescence (ECL) Plus substrate was purchased from Beyotime Institute of Biotechnology.
Cell culture
RPMI-1640 medium was mixed with 10% fetal bovine serum (FBS), 100 U/mL penicillin and 100 μg/mL streptomycin (Hangzhou). Then both Nalm-6 and Jurkat cells were cultured with the mixed medium at 37°C in a 5% CO2 humidified atmosphere.
Cell viability assay
A CCK-8 assay was used to assess the effects of HQH on the proliferation of Jurkat and Nalm-6 leukemia cells. Briefly, cells in the exponential growth phase were seeded in a 96-well plate at 100 μL/well (3×104 to 5×104 cells/mL) and 3 wells were used per group. After incubation for 4 hours, cells were cultured with various concentrations of HQH (1, 5, 10, and 20 mg/mL) for 24, 48, or 72 hours or different concentrations (1, 10, 100, 500, and 1000 μmol/L) of DEX for 24 hours. A total of 10 μL of CCK-8 solution was added to each well following treatment with HQH and cells were incubated for a further 4 hours. The WST-8(2-(2- methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfonyl benzene)-2 h-tetrazolium monosodium salt) formazan product was detected by the microplate reader (Tecan Group, Ltd.) at 490 nm. The following formula was used to calculate the viability ratio of ALL cells. Viability ratio=[(absorbance of experimental group–absorbance of blank group)/(absorbance of untreated group–absorbance of blank group)]×100% [23]. Each experiment was repeated for 3 times.
Apoptosis
1×105 cells in the logarithmic growth phase were seeded in a well in a 6-well plate and treated with HQH (0, 1, 5, 10, or 20 mg/mL). The cells were collected after 24 hours of treatment, washed twice with cold phosphate-buffered saline (PBS) and resuspended with binding buffer to form a final concentration of 1×106 cells/100 μL containing 5 μg/mL FITC Annexin V and 5 μg/mL PI. Cells were cultured without light at room temperature. 15 minutes later, each reaction tube was added with 400 μL of binding buffer. Apoptosis of cells was analyzed with a FACScan™ flow cytometer (BD Biosciences).
RT-qPCR
After treatment with DEX, HQH or a combination of DEX and HQH for 24 hours, total RNA was extracted from Nalm-6 and Jurkat cells using RNAiso Plus, according to the manufacturer’s protocol. Total RNA samples were reversely transcribed to cDNA using PrimeScript RT Master mix. qPCR was performed on a StepOnePlus Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.) using TB Green Premix Ex Taq. PCR products were analyzed using StepOne Software version 2.3. Specific primers were purchased from HyCell Biotechnology and the sequences are listed in Table 1. Relative gene expression differences were calculated using the 2−ΔΔCq method and normalized to GAPDH.
Table 1.
Primer sequences.
| H-GRα | |
| Forward | CTATGCATGAAGTGGTTGAAAA |
| Reverse | TTTCAGCTAACATCTCGGG |
| H-GAPDH | |
| Forward | GAAGCTTGTCATCAATGGAAAT |
| Reverse | TGATGACCCTTTTGGCTCCC |
| H-GILZ | |
| Forward | CAGTGAGCAACTTTCGGCAG |
| Reverse | CATGGTCTGGTCGATGTTGC |
| H-NFKBIA | |
| Forward | CCACTCCATCCTGAAGGCTAC |
| Reverse | CCTGAGCATTGACATCAGCAC |
Western blot analysis
Cells in the logarithmic growth phase were seeded into 6-well plates with DEX, HQH or a co-treatment of DEX and HQH for 24 hours. Following treatment, cells were harvested, washed with PBS and lysed in lysis buffer (Beyotime Institute of Biotechnology). Bicinchoninic acid assay kit (Biosharp) was used to determine protein concentration following the manufacturer’s protocol. Samples were boiled for 10 minutes at 95°C and then allowed to cool to room temperature. The same amount of protein (30–50 μg/lane) was loaded on a 10% SDS-PAGE gel (Beyotime Institute of Biotechnology) and subsequently transferred to a PVDF membrane (EMD Millipore). Membranes were blocked in 5% nonfat milk and incubated at 4°C overnight with a primary antibody against GRα, GRβ, pERK, total ERK, BAX, Bcl-2, cleaved-caspase-3, β-actin or histone H3 (all used at 1: 1000 dilution), and subsequently incubated with the appropriate HRP-conjugated secondary antibody (1: 5000). Immunoreactive bands were visualized using ECL and imaged using a UVP Biospectrum 600 (UVP LLC). The loading control was β-actin.
Immunofluorescence
After co-culture for 24 hours with 4.3 mg/mL HQH for Jurkat cells and 1.5 mg/mL HQH for Nalm-6 cells, cells were centrifuged, removed from the medium, and then fixed with 4% paraformaldehyde. ALL cells were washed for 3 times, smeared and permeated with 0.5% Triton x-100 for 20 minutes at room temperature. ALL cells were blocked with normal goat serum at room temperature for 30 minutes, and then incubated with 1: 100 GRα overnight at 4°C. ALL cells were washed for 3 times and incubated with fluorescein conjugated goat anti-rabbit IgG (1: 100) in the dark at 37°C for 1 hour followed by incubation with DAPI out of light for 5 minutes. A fluorescent microscope (Olympus, BX53) was used to view the results.
Statistical analysis
Data was expressed as the mean±standard deviation, and experiments were repeated for 3 times. Statistical comparisons were performed in GraphPad Prism version 7.0 (GraphPad Software, Inc.). Differences in the drug response were analyzed using a Student’s t-test and a one-way ANOVA. P<0.05 was statistically significant.
Results
HQH accentuated the inhibitory effects of DEX on cell proliferation in ALL cells
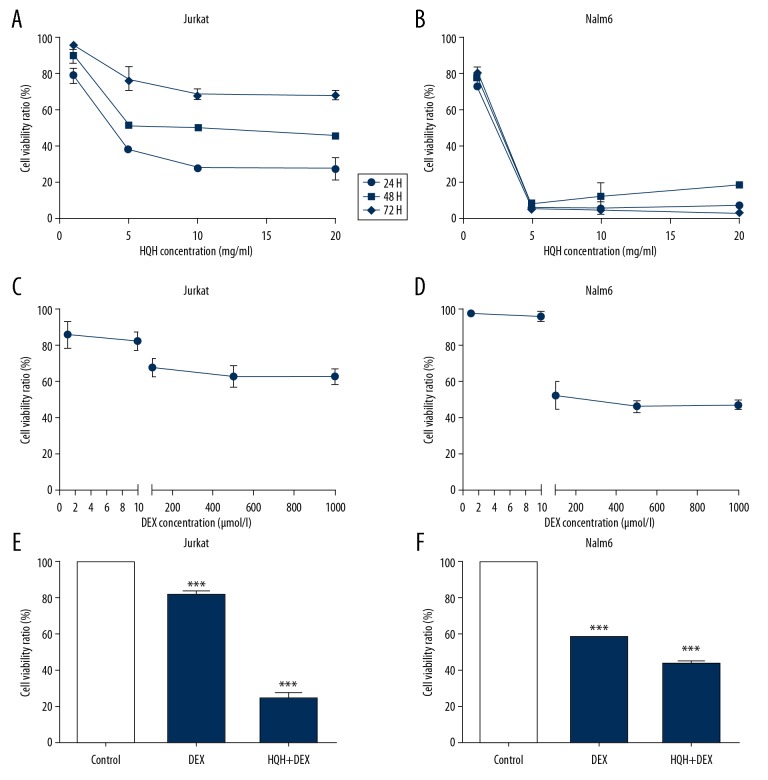
To determine the potential of HQH to decrease the viability of ALL cells, human T-ALL cell line (i.e., Jurkat) and human B-ALL cell line (i.e., Nalm 6) [24] were cultured with various concentrations of HQH ointment (1, 5, 10, and 20 mg/mL) for 24, 48, and 72 hours to determine the most appropriate treatment concentration and time, and a CCK-8 assay was used to determine cell viability. As shown in Figure 1, 24 hours was the most suitable time for HQH to inhibit the cell proliferation of both Jurkat (Figure 1A) and Nalm-6 (Figure 1B) cells, and the IC50 was 4.3 mg/mL and 1.5 mg/mL for Jurkat and Nalm-6 cells, respectively. To determine the effects of DEX on the viability of ALL cells, different concentrations (1, 10, 100, 500, and 1000 μmol/L) of DEX were used to treat Jurkat (Figure 1C) and Nalm-6 (Figure 1D) cells for 24 hours. A minimum concentration of 100 μmol/L DEX significantly inhibited cell growth in both cells. However, further increasing DEX concentration did not notably decrease viability further compared with 100 μmol/L. To determine the effects of combination of HQH and DEX on cell viability, cells were treated with 100 μmol/L DEX and 4.3 mg/mL HQH for Jurkat cells and 1.5 mg/mL HQH for Nalm-6 cells for 24 hours. The results showed that HQH accentuated the effects of DEX on both Jurkat (Figure 1E) and Nalm-6 cells (Figure 1F). (P<0.001).
Figure 1.
HQH enhances the effects of DEX-induced decrease in cell viability. Increasing concentrations of HQH decreased cell viability of Jurkat (A) and Nalm-6 (B) cells. Cell viability of Jurkat (C) and Nalm-6 (D) cells was shown treated with different concentrations of DEX for 24 hours. Jurkat (E) and Nalm-6 cells (F) were co-cultured with 100 μmol/L DEX and 4.3 mg/mL HQH for Jurkat cells and 1.5 mg/mL HQH for Nalm-6 cells for 24 hours. *** P<0.001. HQH – Huai Qi Huang; DEX – dexamethasone.
HQH induced apoptosis in ALL cells
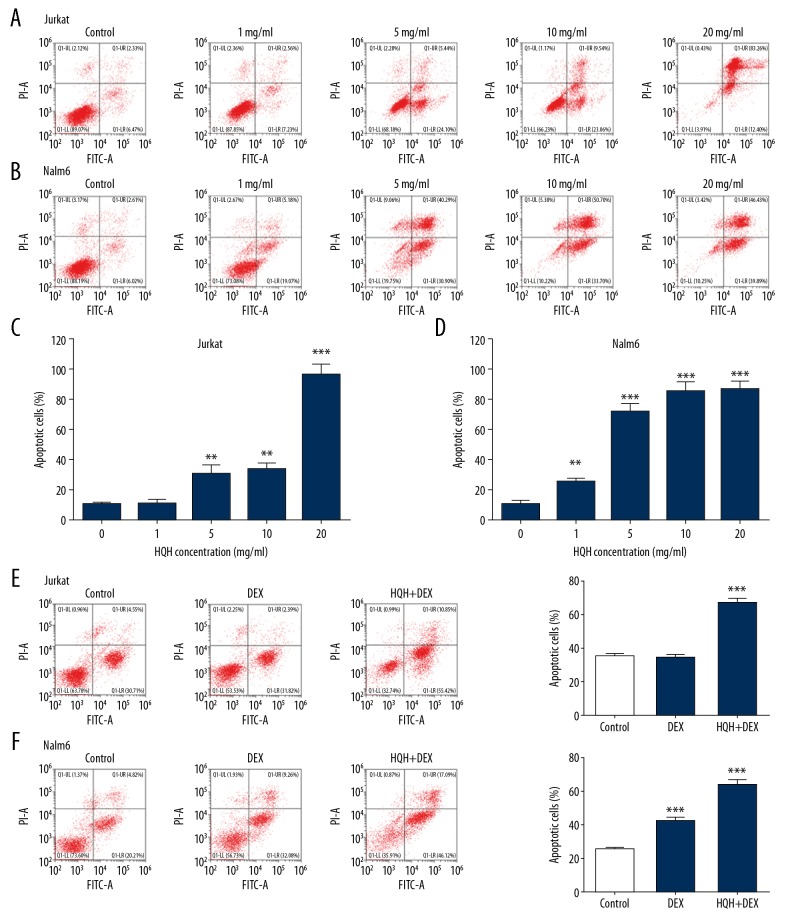
Jurkat and Nalm-6 cells were stained using Annexin V-FITC and PI. Then apoptosis of ALL was detected by flow cytometry. Jurkat (Figure 2A) and Nalm-6 (Figure 2B) cells were cultured with different doses (1, 5, 10, and 20 mg/mL) of HQH for 24 hours. As shown in Figure 2, the increase of apoptotic rate of ALL cells was dose-dependent in both Jurkat (Figure 2C) and Nalm cells (Figure 2D). To demonstrate the synergistic pro-apoptotic effect of HQH and DEX, we performed a co-treatment of 100 μmol/L DEX and 4.3 mg/mL HQH for Jurkat cells (Figure 2E) and 1.5 mg/mL HQH for Nalm-6 cells (Figure 2F), and the results showed that combination therapy was more effective in promoting apoptosis than DEX alone (P<0.01 or P<0.001).
Figure 2.
HQH enhances DEX-induced apoptosis in Jurkat and Nalm-6 cells. Flow cytometry dot-plots were shown for Jurkat (A) and Nalm-6 (B) cells cultured with various concentrations of HQH for 24 hours. Bar graphs were shown for quantification of apoptosis for Jurkat (C) and Nalm-6 (D) cells cultured with various concentrations of HQH for 24 hours. A co-treatment of 100 μmol/L DEX and 4.3 mg/mL HQH for Jurkat cells (E) and 1.5 mg/mL HQH for Nalm-6 cells (F) was performed, and the apoptosis bar graphs were right adjacent to the dot-plots. ** P<0.01, *** P<0.001. HQH – Huai Qi Huang; DEX – dexamethasone; FITC – fluorescein isothiocyanate; PI – propidium iodide.
HQH promoted cell death in ALL cells through upregulation of GRα and downregulation of pERK
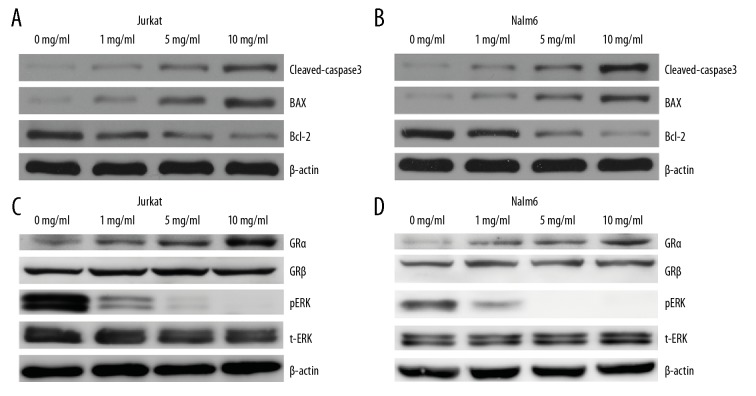
To further evaluate HQH-induced apoptosis on ALL cells, various concentrations of HQH (1, 5, and 10 mg/mL) were used to treat cells for 24 hours, and BAX, Bcl-2, and cleaved-caspase-3 were determined by western blotting. The results demonstrated that BAX and cleaved-caspase-3 were upregulated along with downregulation of Bcl-2. The results were dose-dependent in Jurkat and Nalm-6 cells (Figure 3A, 3B, respectively).
Figure 3.
HQH promotes apoptosis of ALL cells by upregulating GRα and downregulating pERK. BAX and cleaved-caspase-3 were upregulated and Bcl-2 was downregulated in both Jurkat (A) and Nalm-6 (B) cells. Additionally, HQH increased apoptosis by upregulating GRα and downregulating pERK levels in Jurkat (C) and Nalm-6 (D) cells. The loading control was β-actin. HQH – Huai Qi Huang; ALL – acute lymphoblastic leukemia; pERK – phospho-ERK; GR – glucocorticoid receptor; t-ERK – total ERK.
In order to explore the mechanism of HQH promoting apoptosis of ALL cells, we detected changes of GRα, GRβ, total ERK (t-ERK) and pERK using western blotting. HQH increased the expression of GRα, but not GRβ, and the results were dose-dependent in both Jurkat and Nalm-6 cells (Figure 3C, 3D, respectively). Meanwhile, pERK expression decreased, whereas t-ERK expression levels did not change significantly.
HQH increased the sensitivity of ALL cells to DEX
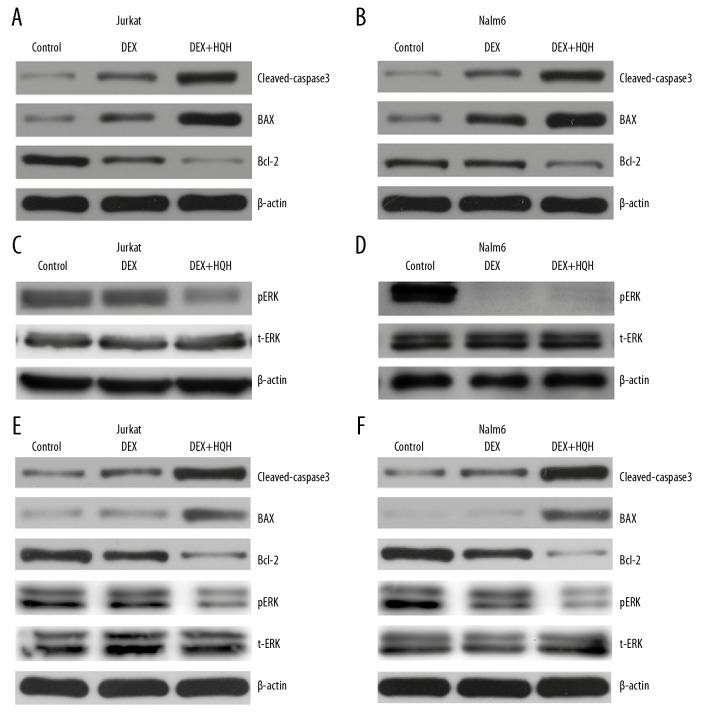
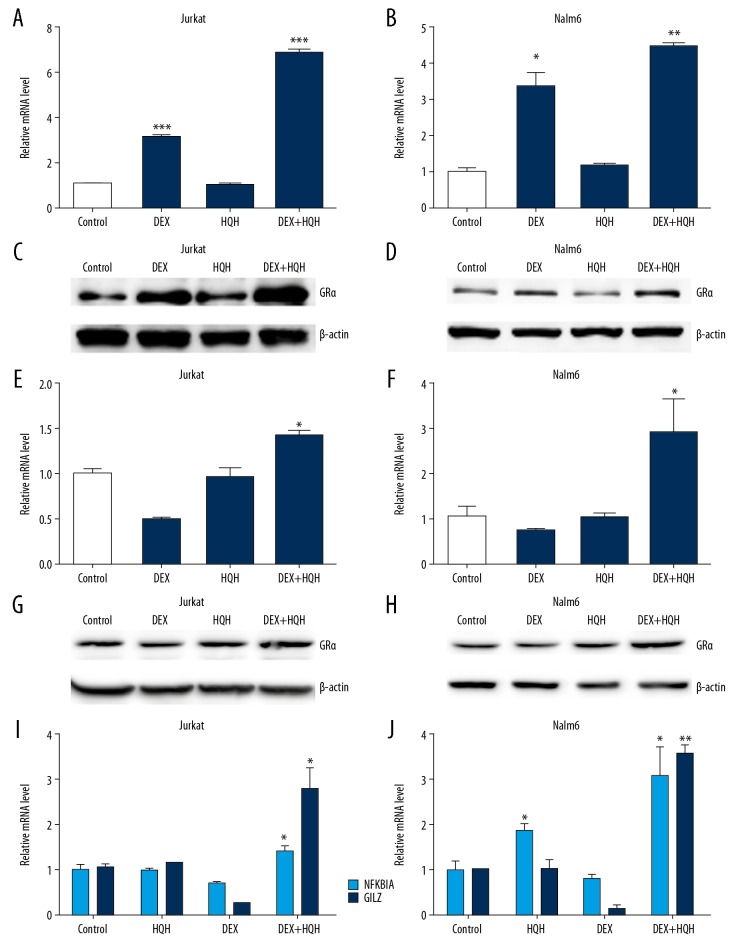
Jurkat and Nalm-6 cells were cultured with 100 μmol/L DEX alone or combined with 4.3 mg/mL HQH for Jurkat cells and 1.5 mg/mL HQH for Nalm-6 cells for 24 hours. Changes of BAX, cleaved-caspase-3, Bcl-2, GILZ, NFKBIA, total ERK (t-ERK), pERK and GRα were measured using western blotting and RT-qPCR. As shown in Figures 4 and 5, DEX combined with HQH was more effective than DEX alone in increasing apoptosis in Jurkat and Nalm-6 cells as determined by upregulation of BAX, cleaved-caspase-3 and GRα, and downregulation of Bcl-2 and pERK (Figures 4A–4D, 5A–5D), although total ERK did not change significantly at protein levels (Figure 4C, 4D). These results suggest that HQH significantly augmented the DEX-induced apoptosis by upregulation of GRα and downregulation of pERK in Jurkat and Nalm-6 cells. HQH combined with 100 nM DEX also increased the lethality of DEX against ALL with upregulation of BAX, cleaved-caspase-3, Gilz, NFKBIA and GRα, and downregulation of Bcl-2 and pERK (Figures 4E, 4F, 5E–5J).
Figure 4.
Co-treatment of cells with DEX and HQH enhances BAX and cleaved-caspase-3 expression and reduces Bcl-2 and pERK expression. Cells were cultured with 100 μmol/L DEX alone or DEX combined with 4.3 mg/mL HQH for Jurkat cells and 1.5 mg/mL HQH for Nalm-6 cells for 24 hours. We used western blotting to detect protein expression levels of BAX, cleaved-caspase-3, Bcl-2, t-ERK and pERK in Jurkat (A, C) and Nalm-6 (B, D) cells. 100 nmol/L DEX and the same dose of HQH as above were used to treat ALL cells for 24 hours, and western blotting was used to detect protein expression levels of BAX, cleaved-caspase-3, Bcl-2, t-ERK and pERK in Jurkat (E) and Nalm-6 (F) cells. The loading control was β-actin. DEX – dexamethasone; HQH – Huai Qi Huang; pERK – phospho-ERK; t-ERK – total ERK.
Figure 5.
Combined treatment with DEX and HQH is more effective in upregulating GRα compared with DEX alone. Jurkat (A, C) and Nalm-6 (B, D) cells were cultured using 100 μmol/L DEX, 4.3 mg/mL HQH for Jurkat cells and 1.5 mg/mL HQH for Nalm-6 cells or DEX combined with HQH for 24 hours. GRα expression at mRNA level (A, B) and protein level (C, D) was measured. 100 nmol/L DEX and the same dose of HQH as above were used to treat Jurkat (E, G, I) and Nalm-6 (F, H, J) cells for 24 hours. The mRNA level of GRα (E, F), NFKBIA and GILZ (I, J) was measured by RT-qPCR and the protein level of GRα (G, H) was measured by western blotting. β-actin and GAPDH were used as the internal control for western blotting and reverse transcription-quantitative PCR, respectively. * P<0.05, ** P<0.01, *** P<0.001. HQH – Huai Qi Huang; DEX – dexamethasone; GR – glucocorticoid receptor; RT-qPCR – reverse transcription-quantitative PCR.
HQH and PD98059 exhibited synergistic effects in promoting cell death
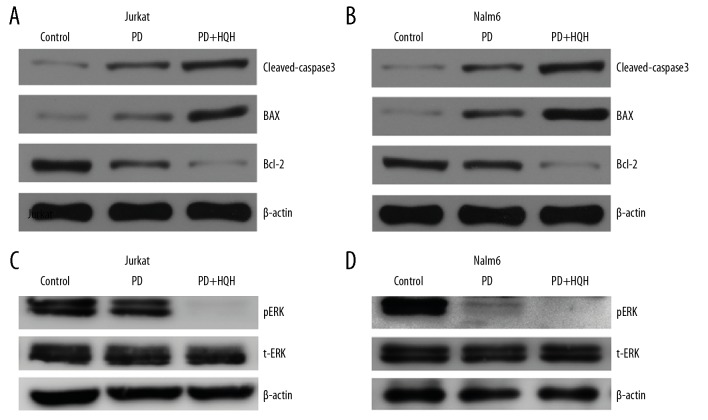
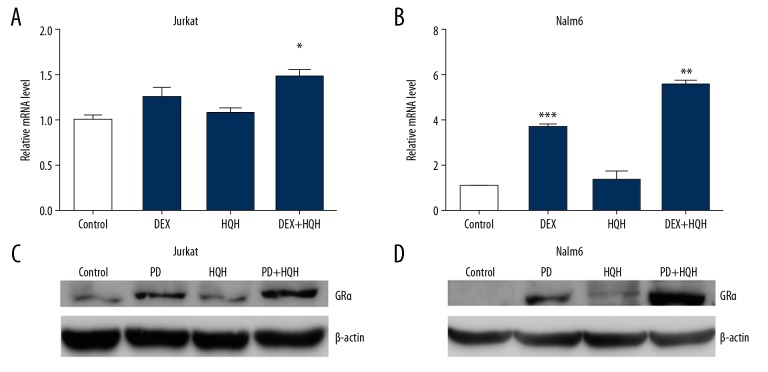
To determine whether HQH induced apoptosis through the MEK/ERK pathway, Jurkat and Nalm-6 cells were cultured with 50 μmol/L of MEK inhibitor (PD98059) alone or with a co-treatment of 50 μmol/L of PD98059 and 4.3 mg/mL HQH for Jurkat cells and 1.5 mg/mL HQH for Nalm-6 cells. Protein expression levels were determined after treatment for 24 hours. As shown in Figure 6, co-treatment of PD98059 and HQH enhanced the inhibition of MEK/ERK and promoted apoptosis in Jurkat and Nalm-6 cells compared with PD98059 alone, and this was accompanied with upregulation in the levels of BAX and cleaved-caspase-3 and downregulation of Bcl-2 and pERK levels. However, there were no significant changes for total ERK in protein levels. Pre-treatment of cells with PD98059 prior to treatment with HQH increased GRα expression on mRNA and protein levels (Figure 7). These results suggest that HQH promoted apoptosis through downregulation of the MEK/ERK pathway and upregulation of GRα expression in Jurkat and Nalm-6 cells.
Figure 6.
HQH inhibits ERK phosphorylation through inhibition of MEK in Jurkat and Nalm-6 cells. ALL cells were cultured with 50 μmol/L of the MEK inhibitor PD98059 alone, or combination of 4.3 mg/mL HQH for Jurkat cells and 1.5 mg/mL HQH for Nalm-6 cells and PD98059 for 24 hours. We used western blotting to detect the proteins of cleaved-caspase 3, BAX, Bcl-2, t-ERK and pERK in Jurkat (A, C) and Nalm-6 (B, D) cells. β-actin was used as the internal control for western blotting. PD – PD98059; ALL – acute lymphoblastic leukemia; HQH – Huai Qi Huang; pERK – phospho-ERK; t-ERK – total ERK.
Figure 7.
Inhibition of the MEK/ERK pathway enhances GRα expression. ALL cells were cultured with either 50 μmol/L PD98059, 4.3 mg/mL HQH for Jurkat cells and 1.5 mg/mL HQH for Nalm-6 cells or PD98059 combined with HQH. RT-qPCR and western blotting were used to explore the mRNA and protein expression levels of GRα after treatment for 24 hours, respectively, in Jurkat (A, C) and Nalm-6 (B, D) cells. β-actin was used as the loading control for western blotting and GAPDH was used as the internal control for RT-qPCR. * P<0.05, ** P<0.01, *** P<0.001. PD – PD98059; ALL – acute lymphoblastic leukemia; HQH – Huai Qi Huang; RT-qPCR – reverse transcription-quantitative PCR; GR – glucocorticoid receptor.
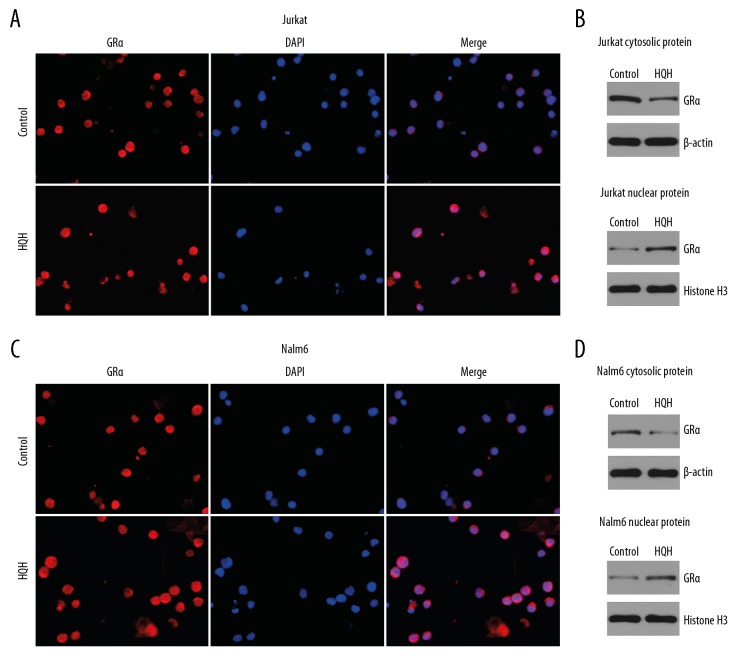
HQH promoted translocation of glucocorticoid receptor α in ALL cells
To demonstrate that HQH can promote the transfer of GRα from cytoplasm to nucleus, ALL cells were treated with 4.3 mg/mL HQH for Jurkat cells and 1.5 mg/mL HQH for Nalm-6 cells for 24 hours. Immunofluorescence was used to show the subcellular localization of GRa. Western blotting was used to explore the GRα of cytoplasm and nucleus. As shown in Figure 8, HQH promoted the transfer of GRα from the cytoplasm to the nucleus, increased the expression of nuclear GRα and decreased the expression of cytoplasmic GRα in Jurkat (Figure 8A, 8B) and Nalm-6 cells (Figure 8C, 8D).
Figure 8.
HQH promotes translocation of GRα. Immunofluorescence showed GRα expression in Jurkat cells (A) treated with 4.3 mg/mL HQH and Nalm-6 cells (C) treated with 1.5 mg/mL HQH and control groups. HQH increased the expression of GRα in the nucleus and decreased the expression of GRα in the cytoplasm in Jurkat (B) and Nalm-6 cells (D). Western blotting was used to test the protein expression levels. β-actin and histone H3 were used as the internal control for cytosolic protein and nuclear protein, respectively. HQH – Huai Qi Huang; DEX – dexamethasone; GR – glucocorticoid receptor.
Discussion
Because of drug resistance, side effects, and the high cost of traditional western treatments [25], medicinal plants or herbs have been used as alternative anti-cancer drugs and have attracted attention due to their relatively low toxicity and cost, and enhanced efficacy in recent years [26–28]. Recently, there has been increasing evidence demonstrating the anti-cancer effects of Huaier, such as the anti-proliferative effects, induction of apoptosis and anti-metastatic effects [13,29–33]. The results of the present study showed that HQH inhibited the proliferation and promoted the apoptosis of the Jurkat and Nalm-6 cells.
Apoptosis is the result of interactions between pro-apoptotic factors and anti-apoptotic factors. When BAX, a pro-apoptotic protein, is dominant, apoptosis is induced. Otherwise, Bcl-2 inhibits apoptosis, allowing the cell to continue surviving. Mitochondrial dysfunction and caspase activation are downstream events during apoptosis of a cell [34]. The final part of the signaling pathway in an apoptosing cell is the activation of caspases. As an effector and executioner caspase, caspase-3 is activated by caspase-8, and subsequently cleaves different substrates [35,36]. Zhang et al. [29], Yan et al. [30], and Zhang et al. [31] showed that Huaier extract promoted apoptosis, as demonstrated by upregulating BAX and caspase-3 and downregulating Bcl-2. The Bcl-2 expression was suppressed, and the expression of BAX was upregulated in MCF-7 cells treated with 4 mg/mL and 8 mg/mL Huaier aqueous extract for 48 hours and 72 hours [31]. The results of the present study showed that HQH also increased apoptosis as BAX and cleaved-caspase-3 levels were upregulated and Bcl-2 levels were downregulated.
As a widely distributed steroid hormone, glucocorticoids bind to GRs, which are ubiquitously expressed. GR, which is bound to and activated by steroid ligands, activates or inhibits glucocorticoid-mediated gene transcription [37,38]. There are 5 known GR isoforms: GRα, GRβ, GRγ, GRp, and GRA [7]. DEX, a widely used glucocorticoid agonist, binds to GRα, and regulates cellular functions. GRβ, which exists as a negative inhibitor of GRα activity, does not bind glucocorticoids [39]. Several studies have revealed that increasing GR expression enhanced the sensitivity of glucocorticoid-resistant ALL cells to glucocorticoids [40–45]. The results of present study showed that HQH enhanced the expression of GRα, but not GRβ in Jurkat and Nalm-6 cells. The combination of HQH and DEX exhibited synergistic effects on the upregulation of GRα compared with DEX alone and up-regulated Gilz and NFKBIA. Nicholson et al. [46] reported that upregulation of GILZ and NFKBIA (IκBα gene) increased glucocorticoid sensitivity and inhibited cell proliferation. Upregulation of NFKBIA induced apoptosis in both LN229 and U87MG cells [47]. Although Riml et al. [48] believed that glucocorticoid resistance was caused by GR auto-induction absence rather than the decrease in basal GR levels in Jurkat cells, Ledderose et al. [49] and Li et al. [50] reported that sensitivity to glucocorticoids could be promoted by increasing GR, which was consistent with our opinion.
Glucocorticoid resistance is complex, and there are other mechanisms besides the decrease of GR. Poulard et al. [51,52] reported that inhibiting Aurora kinase B (AURKB) or increasing G9a auto-methylation could enhance sensitivity of glucocorticoid-resistant B-ALL to glucocorticoids therapy. Shi et al. [53] reported that the NUP98-HOXD13 fusion oncogene could promote T-ALL.
In ALL and acute myelogenous leukemia (AML), the MEK/ERK pathway is frequently activated, resulting in proliferation. Downregulation of ERK activity by MEK/ERK inhibitors induces apoptosis and inhibits proliferation of ALL cells and primary AML blasts [54,55]. PD98059, a MEK inhibitor [56], induced a decrease in blast cell proliferation and an increase in apoptosis by reducing the levels of ERK1/2 activity [54,57,58]. Rambal et al. demonstrated that 50 μM PD98059 significantly inhibited the phosphorylation of ERK, resulting in a decrease in pERK levels in CEM lymphoblast cells [55]. In the present study, phosphorylation of ERK was significantly decreased in cells treated with HQH. Co-treatment of cells with HQH and PD98059 reduced pERK levels, enhanced GRα levels and exhibited synergistic effects. Except the MEK/ERK pathway, other pathways have been involved in the apoptosis of leukemia cells. Bortolozzi et al. [59] revealed that AMPK-mTOR pathway played an important role in T-ALL cell death. Lv et al. [60] reported that c-Myc-CDK4/CDK6 axis was important in B-ALL and T-ALL cell apoptosis.
Conclusions
In summary, the present study is the first study to demonstrate that combined treatment of HQH with DEX significantly promoted apoptosis of lymphoblastic leukemia cells by upregulating expression of GRα and inhibiting the MEK/ERK pathway in vitro, to the best of our knowledge. Therefore, HQH may be used as an adjuvant therapy in patients with ALL.
Acknowledgements
We would like to thank Qidong Gaitianli Medicine Co., Ltd. for gifting our laboratory with the HQH electuary ointment.
Footnotes
Source of support: The present study was supported by a grant from the National Natural Science Foundation of China (grant no. 81700147)
Conflict of interests
None.
References
- 1.Raetz EA, Teachey DT. T-cell acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2016;2016:580–88. doi: 10.1182/asheducation-2016.1.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carroll WL, Raetz EA. Clinical and laboratory biology of childhood acute lymphoblastic leukemia. J Pediatr. 2012;160:10–1. doi: 10.1016/j.jpeds.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Lv M, Wang Y, Wu W, et al. CMyc inhibitor 10058F4 increases the efficacy of dexamethasone on acute lymphoblastic leukemia cells. Mol Med Rep. 2018;18:421–28. doi: 10.3892/mmr.2018.8935. [DOI] [PubMed] [Google Scholar]
- 4.Langebrake C, Reinhardt D, Ritter J. Minimising the long-term adverse effects of childhood leukemia therapy. Drug Saf. 2002;25:1057–77. doi: 10.2165/00002018-200225150-00002. [DOI] [PubMed] [Google Scholar]
- 5.Pieters R, den Boer ML, Durian M, et al. Relation between age, immunophenotype and in vitro drug resistance in 395 children with acute lymphoblastic leukemia – implications for treatment of infants. Leukemia. 1998;12:1344–48. doi: 10.1038/sj.leu.2401129. [DOI] [PubMed] [Google Scholar]
- 6.Schmiegelow K, Nyvold C, Seyfarth J, et al. Post-induction residual leukemia in childhood acute lymphoblastic leukemia quantified by PCR correlates with in vitro prednisolone resistance. Leukemia. 2001;15:1066–71. doi: 10.1038/sj.leu.2402144. [DOI] [PubMed] [Google Scholar]
- 7.Tissing WJ, Meijerink JP, den Boer ML, Pieters R. Molecular determinants of glucocorticoid sensitivity and resistance in acute lymphoblastic leukemia. Leukemia. 2003;17:17–25. doi: 10.1038/sj.leu.2402733. [DOI] [PubMed] [Google Scholar]
- 8.Platanias LC. Map kinase signaling pathways and hematologic malignancies. Blood. 2003;101:4667–79. doi: 10.1182/blood-2002-12-3647. [DOI] [PubMed] [Google Scholar]
- 9.Hsu SC. Involvement of p38 mitogen-activated protein kinase in different stages of thymocyte development. Blood. 2003;101:970–76. doi: 10.1182/blood-2002-03-0744. [DOI] [PubMed] [Google Scholar]
- 10.Huang S, Jiang Y, Li Z, et al. Apoptosis signaling pathway in T cells is composed of ICE/Ced-3 family proteases and MAP kinase 6b. Immunity. 1997;6:739–49. doi: 10.1016/s1074-7613(00)80449-5. [DOI] [PubMed] [Google Scholar]
- 11.Thatte U, Bagadey S, Dahanukar S. Modulation of programmed cell death by medicinal plants. Cell Mol Biol (Noisy-le-grand) 2000;46:199–214. [PubMed] [Google Scholar]
- 12.Vickers A. Botanical medicines for the treatment of cancer: Rationale, overview of current data, and methodological considerations for phase I and II trials. Cancer Invest. 2002;20:1069–79. doi: 10.1081/cnv-120005926. [DOI] [PubMed] [Google Scholar]
- 13.Song X, Li Y, Zhang H, Yang Q. The anticancer effect of Huaier (Review) Oncol Rep. 2015;34:12–21. doi: 10.3892/or.2015.3950. [DOI] [PubMed] [Google Scholar]
- 14.Sun Y, Sun T, Wang F, et al. A polysaccharide from the fungi of Huaier exhibits anti-tumor potential and immunomodulatory effects. Carbohydr Polym. 2013;92:577–82. doi: 10.1016/j.carbpol.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Wu T, Chen W, Liu S, et al. Huaier suppresses proliferation and induces apoptosis in human pulmonary cancer cells via upregulation of miR-26b-5p. Febs Lett. 2014;588:2107–14. doi: 10.1016/j.febslet.2014.04.044. [DOI] [PubMed] [Google Scholar]
- 16.Li C, Wu X, Zhang H, et al. A Huaier polysaccharide inhibits hepatocellular carcinoma growth and metastasis. Tumour Biol. 2015;36:1739–45. doi: 10.1007/s13277-014-2775-2. [DOI] [PubMed] [Google Scholar]
- 17.Xie HX, Xu ZY, Tang JN, et al. Effect of Huaier on the proliferation and apoptosis of human gastric cancer cells through modulation of the PI3K/AKT signaling pathway. Exp Ther Med. 2015;10:1212–18. doi: 10.3892/etm.2015.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Zhang N, Huo Q, et al. Huaier aqueous extract suppresses human breast cancer cell proliferation through inhibition of estrogen receptor alpha signaling. Int J Oncol. 2013;43:321–28. doi: 10.3892/ijo.2013.1947. [DOI] [PubMed] [Google Scholar]
- 19.Zhang F, Zhang Z, Liu Z. Effects of Huaier aqueous extract on proliferation and apoptosis in the melanoma cell line A875. Acta Histochem. 2013;115:705–11. doi: 10.1016/j.acthis.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 20.Zhang T, Wang K, Zhang J, et al. Huaier aqueous extract inhibits colorectal cancer stem cell growth partially via downregulation of the Wnt/beta-catenin pathway. Oncol Lett. 2013;5:1171–76. doi: 10.3892/ol.2013.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan X, Lyu T, Jia N, et al. Huaier aqueous extract inhibits ovarian cancer cell motility via the AKT/GSK3beta/beta-catenin pathway. PLoS One. 2013;8:e63731. doi: 10.1371/journal.pone.0063731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Q, Shu C, Laurence AD, et al. Effect of Huaier granule on recurrence after curative resection of HCC: A multicentre, randomised clinical trial. Gut. 2018;67:2006–16. doi: 10.1136/gutjnl-2018-315983. [DOI] [PubMed] [Google Scholar]
- 23.Han J, Lin M, Zhou D, et al. Huang Qi Huai granules induce apoptosis in acute lymphoblastic leukemia cells through the Akt/FoxO1 pathway. Cell Physiol Biochem. 2016;38:1803–14. doi: 10.1159/000443119. [DOI] [PubMed] [Google Scholar]
- 24.Deng M, Zha J, Jiang Z, et al. Apatinib exhibits anti-leukemia activity in preclinical models of acute lymphoblastic leukemia. J Transl Med. 2018;16:47. doi: 10.1186/s12967-018-1421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan MH, Ho CT. Chemopreventive effects of natural dietary compounds on cancer development. Chem Soc Rev. 2008;37:2558–74. doi: 10.1039/b801558a. [DOI] [PubMed] [Google Scholar]
- 26.Yu J, Liu H, Lei J, et al. Antitumor activity of chloroform fraction of Scutellaria barbata and its active constituents. Phytother Res. 2007;21:817–22. doi: 10.1002/ptr.2062. [DOI] [PubMed] [Google Scholar]
- 27.Harhaji L, Mijatovic S, Maksimovic-Ivanic D, et al. Anti-tumor effect of Coriolus versicolor methanol extract against mouse B16 melanoma cells: In vitro and in vivo study. Food Chem Toxicol. 2008;46:1825–33. doi: 10.1016/j.fct.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 28.Rashid S, Unyayar A, Mazmanci MA, et al. A study of anti-cancer effects of Funalia trogii in vitro and in vivo. Food Chem Toxicol. 2011;49:1477–83. doi: 10.1016/j.fct.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 29.Sun Y, Sun T, Wang F, et al. A polysaccharide from the fungi of Huaier exhibits anti-tumor potential and immunomodulatory effects. Carbohydr Polym. 2013;92:577–82. doi: 10.1016/j.carbpol.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Zheng J, Li C, Wu X, et al. Huaier polysaccharides suppresses hepatocarcinoma MHCC97-H cell metastasis via inactivation of EMT and AEG-1 pathway. Int J Biol Macromol. 2014;64:106–10. doi: 10.1016/j.ijbiomac.2013.11.034. [DOI] [PubMed] [Google Scholar]
- 31.Zhang F, Zhang Z, Liu Z. Effects of Huaier aqueous extract on proliferation and apoptosis in the melanoma cell line A875. Acta Histochem. 2013;115:705–11. doi: 10.1016/j.acthis.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 32.Yan X, Lyu T, Jia N, et al. Huaier aqueous extract inhibits ovarian cancer cell motility via the AKT/GSK3beta/beta-catenin pathway. PLoS One. 2013;8:e63731. doi: 10.1371/journal.pone.0063731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang N, Kong X, Yan S, et al. Huaier aqueous extract inhibits proliferation of breast cancer cells by inducing apoptosis. Cancer Sci. 2010;101:2375–83. doi: 10.1111/j.1349-7006.2010.01680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Korsmeyer SJ. BCL-2 gene family and the regulation of programmed cell death. Cancer Res. 1999;59:1693–700. [PubMed] [Google Scholar]
- 35.Fesik SW, Shi Y. Structural biology. Controlling the caspases. Science. 2001;294:1477–78. doi: 10.1126/science.1062236. [DOI] [PubMed] [Google Scholar]
- 36.Salvesen GS, Duckett CS. IAP proteins: Blocking the road to death’s door. Nat Rev Mol Cell Biol. 2002;3:401–10. doi: 10.1038/nrm830. [DOI] [PubMed] [Google Scholar]
- 37.Wright AP, Zilliacus J, McEwan IJ, et al. Structure and function of the glucocorticoid receptor. J Steroid Biochem Mol Biol. 1993;47:11–19. doi: 10.1016/0960-0760(93)90052-x. [DOI] [PubMed] [Google Scholar]
- 38.Schwartz JR, Sarvaiya PJ, Vedeckis WV. Glucocorticoid receptor knock down reveals a similar apoptotic threshold but differing gene regulation patterns in T-cell and pre-B-cell acute lymphoblastic leukemia. Mol Cell Endocrinol. 2010;320:76–86. doi: 10.1016/j.mce.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oakley RH, Sar M, Cidlowski JA. The human glucocorticoid receptor beta isoform. Expression, biochemical properties, and putative function. J Biol Chem. 1996;271:9550–59. doi: 10.1074/jbc.271.16.9550. [DOI] [PubMed] [Google Scholar]
- 40.Reichardt HM, Umland T, Bauer A, et al. Mice with an increased glucocorticoid receptor gene dosage show enhanced resistance to stress and endotoxic shock. Mol Cell Biol. 2000;20:9009–17. doi: 10.1128/mcb.20.23.9009-9017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmidt S, Irving JA, Minto L, et al. Glucocorticoid resistance in two key models of acute lymphoblastic leukemia occurs at the level of the glucocorticoid receptor. FASEB J. 2006;20:2600–2. doi: 10.1096/fj.06-6214fje. [DOI] [PubMed] [Google Scholar]
- 42.Malyukova A, Brown S, Papa R, et al. FBXW7 regulates glucocorticoid response in T-cell acute lymphoblastic leukaemia by targeting the glucocorticoid receptor for degradation. Leukemia. 2013;27:1053–62. doi: 10.1038/leu.2012.361. [DOI] [PubMed] [Google Scholar]
- 43.Lv M, Zhang X, Jia H, et al. An oncogenic role of miR-142-3p in human T-cell acute lymphoblastic leukemia (T-ALL) by targeting glucocorticoid receptor-alpha and cAMP/PKA pathways. Leukemia. 2012;26:769–77. doi: 10.1038/leu.2011.273. [DOI] [PubMed] [Google Scholar]
- 44.Gruber G, Carlet M, Turtscher E, et al. Levels of glucocorticoid receptor and its ligand determine sensitivity and kinetics of glucocorticoid-induced leukemia apoptosis. Leukemia. 2009;23:820–23. doi: 10.1038/leu.2008.360. [DOI] [PubMed] [Google Scholar]
- 45.Pui CH, Dahl GV, Rivera G, et al. The relationship of blast cell glucocorticoid receptor levels to response to single-agent steroid trial and remission response in children with acute lymphoblastic leukemia. Leuk Res. 1984;8:579–85. doi: 10.1016/0145-2126(84)90006-7. [DOI] [PubMed] [Google Scholar]
- 46.Nicholson L, Hall AG, Redfern CP, Irving J. NFkappaB modulators in a model of glucocorticoid resistant, childhood acute lymphoblastic leukemia. Leuk Res. 2010;34:1366–73. doi: 10.1016/j.leukres.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 47.Zappavigna S, Cossu AM, Abate M, et al. A hydroquinone-based derivative elicits apoptosis and autophagy via activating a ROS-dependent unfolded protein response in human glioblastoma. Int J Mol Sci. 2019;20(15) doi: 10.3390/ijms20153836. pii: E3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Riml S, Schmidt S, Ausserlechner MJ, et al. Glucocorticoid receptor heterozygosity combined with lack of receptor auto-induction causes glucocorticoid resistance in Jurkat acute lymphoblastic leukemia cells. Cell Death Differ. 2004;11(Suppl 1):S65–72. doi: 10.1038/sj.cdd.4401413. [DOI] [PubMed] [Google Scholar]
- 49.Ledderose C, Mohnle P, Limbeck E, et al. Corticosteroid resistance in sepsis is influenced by microRNA-124 – induced downregulation of glucocorticoid receptor-alpha. Crit Care Med. 2012;40:2745–53. doi: 10.1097/CCM.0b013e31825b8ebc. [DOI] [PubMed] [Google Scholar]
- 50.Li XJ, Luo XQ, Han BW, et al. MicroRNA-100/99a, deregulated in acute lymphoblastic leukaemia, suppress proliferation and promote apoptosis by regulating the FKBP51 and IGF1R/mTOR signalling pathways. Br J Cancer. 2013;109:2189–98. doi: 10.1038/bjc.2013.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Poulard C, Kim HN, Fang M, et al. Relapse-associated AURKB blunts the glucocorticoid sensitivity of B cell acute lymphoblastic leukemia. Proc Natl Acad Sci USA. 2019;116:3052–61. doi: 10.1073/pnas.1816254116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poulard C, Baulu E, Lee BH, et al. Increasing G9a automethylation sensitizes B acute lymphoblastic leukemia cells to glucocorticoid-induced death. Cell Death Dis. 2018;9:1038. doi: 10.1038/s41419-018-1110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shields BJ, Slape CI, Vo N, et al. The NUP98-HOXD13 fusion oncogene induces thymocyte self-renewal via Lmo2/Lyl1. Leukemia. 2019;33:1868–80. doi: 10.1038/s41375-018-0361-0. [DOI] [PubMed] [Google Scholar]
- 54.Lunghi P, Tabilio A, Dall’Aglio PP, et al. Downmodulation of ERK activity inhibits the proliferation and induces the apoptosis of primary acute myelogenous leukemia blasts. Leukemia. 2003;17:1783–93. doi: 10.1038/sj.leu.2403032. [DOI] [PubMed] [Google Scholar]
- 55.Rambal AA, Panaguiton ZL, Kramer L, et al. MEK inhibitors potentiate dexamethasone lethality in acute lymphoblastic leukemia cells through the pro-apoptotic molecule BIM. Leukemia. 2009;23:1744–54. doi: 10.1038/leu.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anjum R, Blenis J. The RSK family of kinases: Emerging roles in cellular signalling. Nat Rev Mol Cell Biol. 2008;9:747–58. doi: 10.1038/nrm2509. [DOI] [PubMed] [Google Scholar]
- 57.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 58.Sebolt-Leopold JS. Development of anticancer drugs targeting the MAP kinase pathway. Oncogene. 2000;19:6594–99. doi: 10.1038/sj.onc.1204083. [DOI] [PubMed] [Google Scholar]
- 59.Mariotto E, Bortolozzi R, Volpin I, et al. EB-3D a novel choline kinase inhibitor induces deregulation of the AMPK-mTOR pathway and apoptosis in leukemia T-cells. Biochem Pharmacol. 2018;155:213–23. doi: 10.1016/j.bcp.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 60.Lv M, Wang Y, Wu W, et al. CMyc inhibitor 10058F4 increases the efficacy of dexamethasone on acute lymphoblastic leukaemia cells. Mol Med Rep. 2018;18:421–28. doi: 10.3892/mmr.2018.8935. [DOI] [PubMed] [Google Scholar]