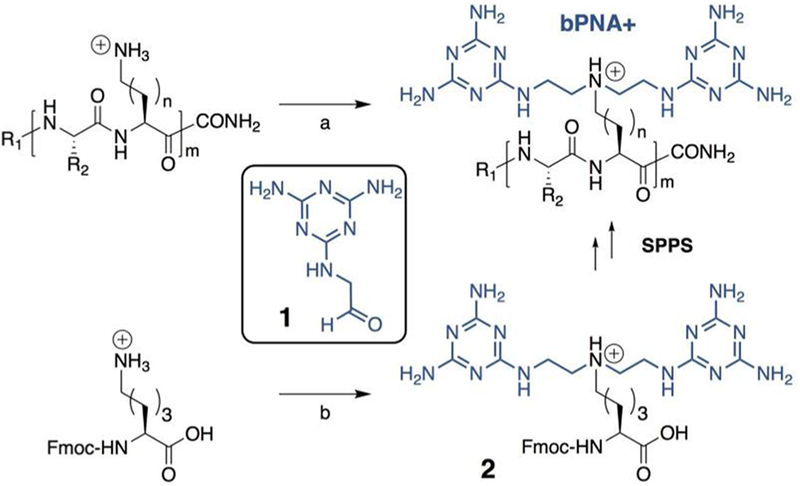
Scheme 1. Reductive Alkylation of Peptides with Amino Side Chains at Alternate Positionsa.
aConditions (a): 1, NaBH3CN, DMSO. Peptides were prepared with Ser (R2=CH2OH) or Glu (R2=(CH2)2CO2H) in the first position and diaminobutyric acid (B, n = 1), ornithine (O, n = 2), and lysine (K, n = 3) in the second position. All peptides were made with m = 3 and m = 5 peptides were prepared with Ser (S) and Glu (E) in the first position and lysine (K) in the second position. (Lower) Fmoc-Lys-OH was prepared as the TFA salt from Fmoc-Lys(Boc) and reductively alkylated to yield compound 2 (Fmoc-K2M–OH) using condition (b): 1, NaBH3CN, MeOH, (83%) and used in standard solid phase peptide synthesis (SPPS) directly to produce the (SK2M)3 and (EK2M)3 peptides.