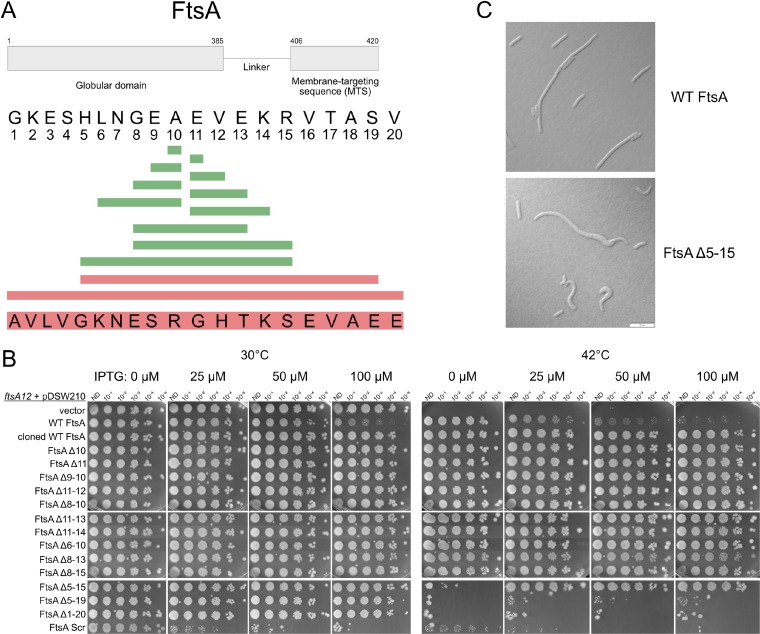
FIG 2.
Effects of deletions within the 20-residue region linking the core oligomeric and amphipathic helix domains of FtsA. (A) Schematic showing the amino acid sequence of the linker and its context within the entire FtsA protein (top), along with the residues deleted that had no effect on viability (green) or that abolished viability (red). The scrambled sequence that replaced the WT sequence is shown at the bottom. (B) After growth at 30°C, ftsA12(Ts) mutant strains containing the pDSW210F plasmid derivatives shown were serially diluted 10-fold and spotted on plates at either the permissive (30°C) or nonpermissive (42°C) temperature with the indicated amounts of IPTG and incubated overnight. The row labeled “cloned WT FtsA” represents a positive-control construct in which the WT linker sequence was cloned into a vector by the same method by which the smaller linker deletions were cloned. (C) Representative micrographs of ftsA null mutant cells harboring pDSW210F expressing either WT FtsA or FtsAΔ5–15 grown at 30°C and induced with 100 μM IPTG, highlighting aberrant cell morphologies. Scale bar, 10 μm.