Figure 3.
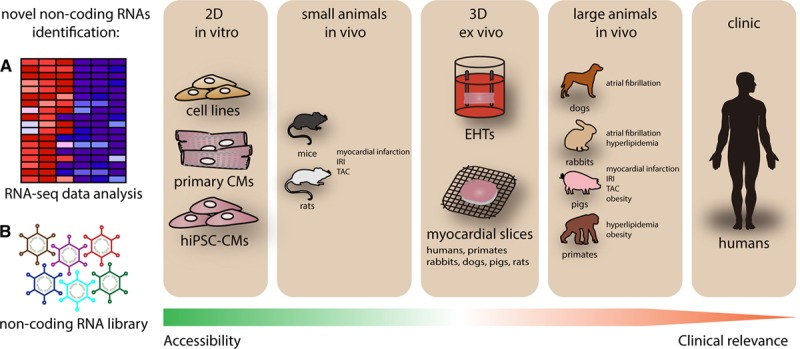
Processes of noncoding RNA (ncRNA)-based drug development. Novel ncRNA candidates are selected from a RNA-seq profile or other ncRNA approaches40,148 and then validated in cardiovascular cells (in vitro). After basic characterization, the ncRNA candidates are further investigated in animal models (in vivo). Several pathophysiological animal models of cardiovascular complication are available in different species, ranging from small to large animals, via the application of surgical techniques, genetic engineering, or diet changes. Some effective yet nontoxic ncRNA candidates are selected for further clinical development. However, successful transitions from preclinical to clinical studies are generally small in number. Often, in vitro models are easy to apply but have limited clinical relevance; while in vivo models have higher clinical relevance, they are challenging to conduct and expensive. To increase the translational efficiency of ncRNA-based therapeutic human induced pluripotent stem cells and/or other human cardiovascular cell types and living myocardial slices, could be powerful tools to bridge the gap between in vivo and clinical development. IRI indicates ischemia-reperfusion injury; and TAC, transverse aortic constriction.
