Abstract
Purpose
The 8th edition of the American Joint Committee on Cancer (AJCC) staging manual introduced a new prognostic staging system for breast cancer. This study aimed to evaluate the changes in staging distribution and predictive power of the new staging system.
Methods
Of the 12,275 patients with breast cancer identified from the Severance Breast Cancer Registry who underwent surgery between 1978 and 2016, 12,125 patients met the inclusion criteria.
Results
In both the 7th and 8th staging systems, stage I patients constituted the largest proportion (38.2% and 48.4%). Migration from the 7th to 8th edition of the AJCC manual resulted in a decrease in stage II population and an increase in stage I and III populations. A total of 1,293 (15.4%) patients were upstaged, and 1,201 (14.3%) were downstaged. Downstaged patients had better recurrence-free and overall survival (p < 0.001). Pathologic complete response after neoadjuvant therapy showed good prognosis as p stage 0, and yp stages I and III showed poorer outcomes than the same p stage (p < 0.001).
Conclusions
Staging migrations are common in early breast cancer under the prognostic staging system. The prognostic staging system of the 8th edition of the AJCC manual discriminates survival outcomes better than the anatomical staging system of the 7th edition of the AJCC manual.
Keywords: Breast neoplasms, Prognostic staging
INTRODUCTION
Cancer staging enables clinicians and researchers to uniformly describe the extent of disease and effectively communicate treatment plans and prognosis [1]. Modern cancer staging of breast cancer is primarily based on the American Joint Committee on Cancer (AJCC) staging system, which was recently revised in 2017 [2].
The AJCC cancer staging manual standardized the TNM classification, which classifies tumors by size, lymph node metastasis, and distal metastasis [3]. The AJCC manual incorporates universal information and understanding of research and is made available to all countries. In addition, the AJCC cancer staging system is applicable to lower and middle-income countries because it requires only basic anatomic information. Hence, clinicians, tumor registrars, patients, and patient advocates use the AJCC staging system worldwide [2,4].
The previous (7th edition) AJCC manual staged cancers solely according to anatomic information. This approach did not consider the remarkable progress of modern molecular biology and the importance of biologic markers, including tumor markers, hormonal-receptor status, histologic findings, and gene expression when planning treatment and predicting cancer prognosis [5,6]. Therefore, a panel of AJCC experts revised the traditional guidelines to include biomarkers. This renewed staging system is the cornerstone of the next-generation AJCC staging system, which is meant to provide a more precise and personalized approach [4].
Major changes to breast cancer staging were incorporated into the 8th edition of the AJCC manual because of the heterogeneous features and diverse biomarkers in breast cancer that can influence treatment plans and prognosis [7]. First, lobular carcinoma in situ (LCIS) was reclassified as a benign lesion and removed from the list of malignant tumor entities. Second, the pathological classification of residual tumor following neoadjuvant chemotherapy (NCT) was clarified and strongly recommended. Third and most importantly, the revision established a completely new prognostic staging table that utilized biomarkers, including histologic grade, estrogen receptor (ER) expression, progesterone receptor (PR) expression, human epidermal growth factor receptor 2 (HER2) expression, and gene expressions [1,6,8].
Therefore, the aim of this study was to investigate the impacts of the revision on the existing stage distributions and assess the predictive power of the revised prognostic staging system.
METHODS
We accessed the Severance Breast Cancer Registry, which was described in a previous study [9], and identified 12,275 patients who underwent definitive surgery for the treatment of breast cancer at Severance Hospital from February 1978 to August 2016. We reviewed the following clinicopathologic data: tumor size; nodal metastasis; distant metastasis; histologic grade; ER, PR, and HER2 expressions; and the 21-gene Oncotype Dx® (Genomic Health, Inc., Redwood City, US). In addition, we reviewed the patients' demographic data, including age, patterns and date of recurrence, and date of death.
The ER and PR status of the patients was determined by an immunohistochemistry (IHC) assay or a ligand-binding assay. Tumors with ≥ 1% nuclear-stained cells were considered positive for ER and PR, and this interpretation is consistent with the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines [10]. HER2 was scored by counting the number of membrane-stained cells, and the positively stained cells were reported as a percentage of the total tumor cells. This interpretation is also consistent with the ASCO/CAP guidelines [9]. Furthermore, HER2 was considered positive if gene amplification was detected by fluorescence in situ hybridization (FISH), regardless of the HER2 IHC results [11]. Previous studies have provided a description of the IHC and FISH testing used in this study [9,11].
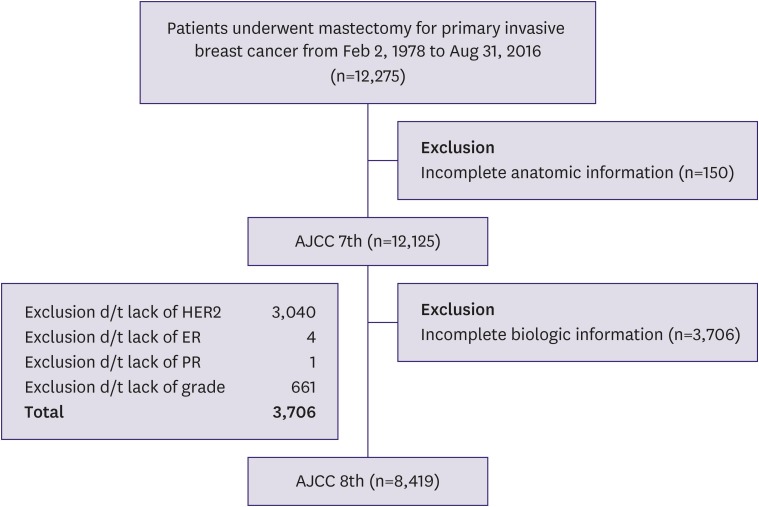
We excluded 150 patients who had incomplete anatomic information. Hence, data from 12,125 patients were analyzed according to the 7th edition of the AJCC manual. We further excluded 3,706 patients who had incomplete prognostic information such as missing hormonal receptor status or histologic grade data. Finally, data from 8,419 patients were analyzed according to the 8th edition of the AJCC manual (Figure 1). When patients were staged with their genomic data (n = 139), we considered the Oncotype Dx® multi-gene assay score. The 8th edition of the AJCC manual had missing subgroups of TNM stages at first [2]. For the missing subgroups, we applied the AJCC software, TNM Cancer Staging Calculator, version 16 (Integrated Cancer Research Ltd., London, UK) to categorize them by the prognostic staging system.
Figure 1. Flow diagram of study cohort.
AJCC = American Joint Committee on Cancer; HER2 = human epidermal growth factor receptor 2; ER = estrogen receptor; PR = progesterone receptor.
Statistical analysis
The intergroup differences were evaluated using the t-test for continuous variables and the χ2 test for categorical variables. We defined overall survival (OS) as the period from the day of operation to the date of death from all causes or date of last visit to the hospital. Recurrence-free survival (RFS) was measured from the day of operation to the date of the first recurrence event, death from any cause, and/-or the last follow up. Survival curves were plotted using the Kaplan-Meier method, and intergroup differences in survival time were investigated using the log-rank test. All statistical tests were 2-sided, and a p-value of < 0.05 was considered statistically significant. SPSS for Windows version 23.0 (IBM Corp., Armonk, USA) was used for all statistical analyses.
The independent Institutional Review Board of Severance Hospital approved this study (approval number: 4-2019-0174), and the need for written informed consents was waived because of the low risk posed by this research.
RESULTS
Patient demographics
The patients' clinicopathologic characteristics are shown in Table 1. Females accounted for 99.3% of the study population, and the median age was 49.0 years (range, 17–93 years). The proportion of patients aged ≤ 50 years was higher than those aged > 50 years. Half of the patients (51.2%) were classified as T1, followed by T2 (28.2%) and Tis (14.4%). In total, 65 patients had only LCIS constituting 3.0% in situ carcinoma according to the 7th edition of the AJCC manual. Further, 70% patients had no regional lymph node metastasis (N0), and the proportion of patients decreased in each successive N stage. Grade 2 histologic tumors were found in 36.4% patients. Similar proportions of patients had grades 1 and 3 tumors (17.4%). More than 60% of the study group was ER positive, and half of the tumors were PR positive. HER2-negative tumors were more common than HER2-positive tumors (52.0% vs. 17.1%), and one-third patients had unknown HER2 status (30.9%). Thirteen percent of patients received preoperative chemotherapy.
Table 1. Characteristics of the study population (n=12,125).
| Characteristics | No. (%) | |
|---|---|---|
| Age (yr) | ||
| ≤ 50 | 6,826 (56.3) | |
| > 50 | 5,298 (43.7) | |
| Sex | ||
| Female | 12,040 (99.3) | |
| Male | 41 (0.3) | |
| Unknown | 44 (0.4) | |
| T stage | ||
| T0 | 286 (2.4) | |
| Tis | 1,759 (14.5) | |
| T1 | 6,201 (51.1) | |
| T2 | 3,414 (28.2) | |
| T3 | 343 (2.8) | |
| T4 | 122 (1.0) | |
| N stage | ||
| N0 | 8,523 (70.3) | |
| N1 | 2,222 (18.2) | |
| N2 | 830 (6.9) | |
| N3 | 533 (4.4) | |
| Unknown | 17 (0.1) | |
| M stage | ||
| M0 | 12,020 (99.1) | |
| M1 | 105 (0.9) | |
| ER | ||
| Negative | 3,462 (28.5) | |
| Positive | 7,458 (61.5) | |
| Unknown | 1,205 (10.0) | |
| PR | ||
| Negative | 4,982 (41.1) | |
| Positive | 5,865 (48.4) | |
| Unknown | 1,278 (10.5) | |
| HER2 | ||
| Negative | 6,299 (52.0) | |
| Positive | 2,077 (17.1) | |
| Unknown | 3,749 (30.9) | |
| Grade | ||
| G1 | 2,103 (17.3) | |
| G2 | 4,425 (36.5) | |
| G3 | 2,116 (17.3) | |
| Unknown | 3,481 (28.7) | |
| NCT | ||
| No | 10,556 (87.1) | |
| Yes | 1,569 (12.9) | |
ER = estrogen receptor; PR = progesterone receptor; HER2 = human epidermal growth factor receptor 2; NCT = neoadjuvant chemotherapy.
Stage distribution and migration
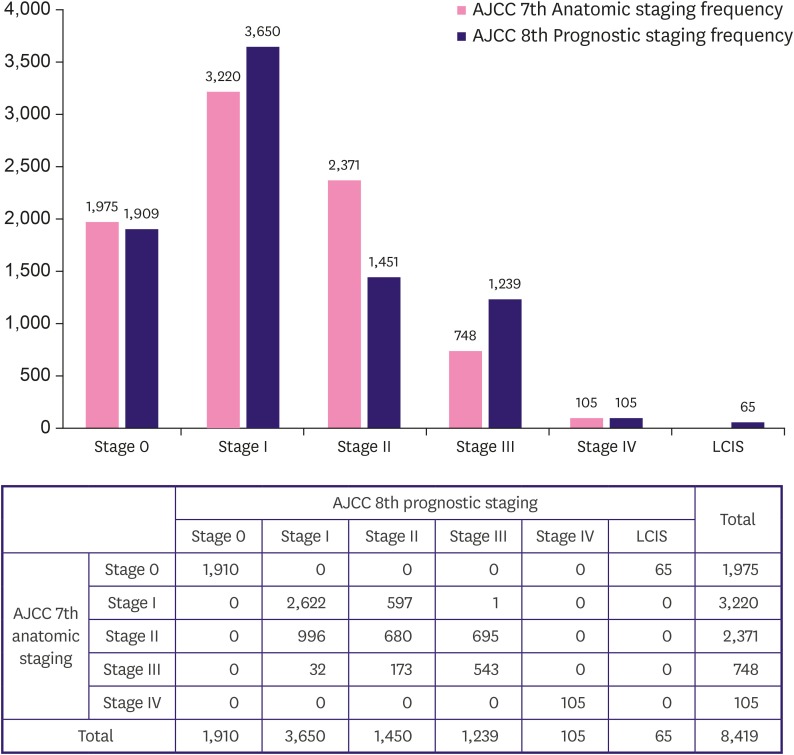
The 7th and 8th editions of the AJCC manual had identical anatomic staging guidelines, except for in situ carcinoma. The study patients’ anatomic staging was distributed as follows: 1,975 (16.3%) were stage 0, 4,598 (37.9%) were stage I, 3,999 (33.0%) were stage II, 1,448 (11.9%) were stage III, and 105 (0.9%) were stage IV. The study patients' prognostic staging was distributed as follows: 1,910 (15.8%) were stage 0, 3,650 (30.1%) were stage I, 1,450 (12.0%) were stage II, 1,239 (10.2%) were stage III, and 105 (0.9%) were stage IV. Figure 2 shows the differences in stage distribution between the two staging systems. The most noticeable changes were the decrease in the stage II population and the increase in the stage I and III populations.
Figure 2. Stage migration of study population.
AJCC = American Joint Committee on Cancer; LCIS = lobular carcinoma in situ as a benign entity.
Figure 2 shows the migration of populations in each stage group. The most frequent movement was from anatomic stage II to prognostic stage I (n = 996), followed by anatomic stage II to prognostic stage III (n = 695), anatomic stage I to prognostic stage II (n = 597), and anatomic stage III to prognostic stage II (n = 173). Thirty-two patients were stage III according to anatomic staging, but the staging was changed to stage I according to the prognostic staging.
Survival analysis according to the staging systems of the 7th and 8th editions of the AJCC manual
The OS and RFS of each stage group were discriminated by the anatomic and prognostic staging systems (p < 0.001). According to the anatomic staging system, the 5- and 10-year OS for each stage is as follows: 97.2% and 93.6% for stage 0, 96.5% and 0, 90.1% for stage I, 91.0% and 81.5% for stage II, 72.5% and 56.7% for stage III, and 40.3% and 12.6% for stage IV, respectively. According to the prognostic staging system, the 5- and 10-year OS for each stage is as follows: 97.3% and 93.6% for stage 0, 97.1% and 90.7% for stage I, 93.0% and 83.4% for stage II, 77.5% and 67.0% for stage III, and 40.3% and 12.6% for stage IV, respectively (Supplementary Table 1). The RFS for each staging system is shown in Supplementary Table 2.
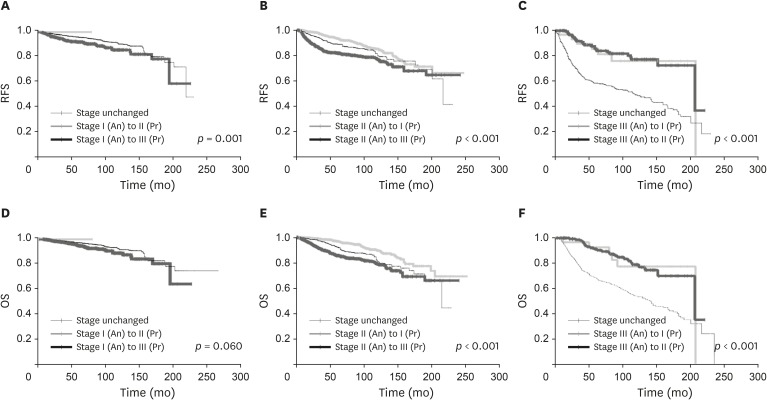
The movement of patients from the anatomic staging system to the prognostic staging system resulted in the upstaging of 1,293 patients and the downstaging of 1,201 patients. The changes in OS and RFS according to upstaging or downstaging are represented in Figure 3. The patients downstaged from stage II to stage I in the prognostic staging system experienced better outcomes, and those upstaged from stage II to stage III in the prognostic staging system had worse outcomes than those who remained in the same stage (p < 0.001). This trend was also observed in stages I and III. There was no significant difference in RFS between the patients downstaged from III to II and those from III to I (p = 0.635).
Figure 3. Changes of RFS (A-C) and OS (D-F) according to migration of anatomic stage (A and D) I, (B and E) II, (C and F) III.
An = anatomic stage according to AJCC 7th edition; Pr = prognostic stage according to AJCC 8th edition; AJCC = American Joint Committee on Cancer; RFS = recurrence-free survival; OS = overall survival.
Survival analysis according to NCT
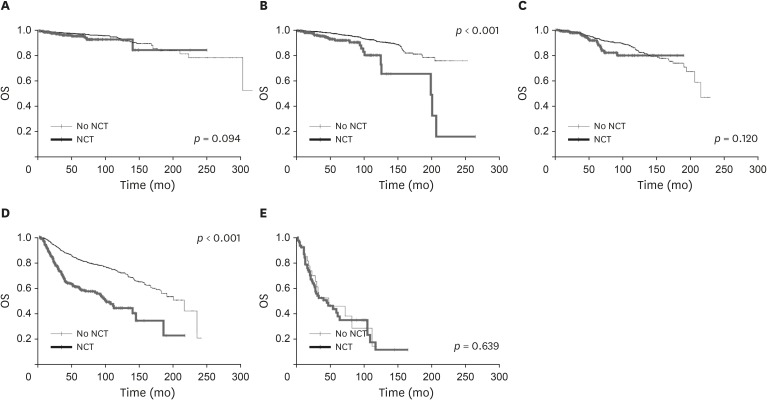
A total of 1,569 patients received NCT. For the patients staged I or III after NCT and subsequent surgery, their prognosis was poorer than those at the same stage without NCT (p < 0.001). The patients at stage 0 after NCT and surgery tended to have worse outcomes than those without NCT, but the trend was not statistically significant (p = 0.065). The prognosis of p- and yp-stages showed no difference for stage II.
DISCUSSION
Our study demonstrates that the prognostic staging system of the 8th edition of the AJCC manual increases the proportion of stages I and III and decreases the proportion of stage II when compared to the anatomical staging system of the 7th edition of the AJCC manual. Our results are consistent with those of previous studies [12,13]. In this study, the change of stage distribution was primarily due to the migration from stage II. The largest migration was from stage II to stage I, which was approaching the sum of the migrations to stage II from stage III and I. Hence, the distribution of the study population became more bipolarized under the prognostic staging system. This suggests that the new guideline distributes the patients more towards good or poor prognosis groups (stage I or III) rather than the equivocal prognosis group (stage II).
The prognostic staging system discriminated survival better than the anatomical staging system. Downstaged patients showed better survival than the unchanged or upstaged patients (Figure 3), a finding consistent with those of previous studies [1,7,12,14]. Thus, the prognostic staging system of the 8th edition of the AJCC manual shows better discrimination of survival outcomes than the anatomical staging system of the 7th edition of the AJCC manual.
This study showed differences in survival outcomes between the patients with recommendations for neoadjuvant pathologic tumor-node-metastasis staging (ypTNM) and those with pathological tumor-node-metastasis staging (pTNM) even though they had the same stages. The patients with stages I and III after NCT (yp stage I and III) showed significantly poorer survival than those with the same stage without NCT. Because the stages after NCT were all residual cases, the worse outcomes may be related to chemoresistance. In general, patients with residual chemo-resistant tumors have poorer survival than those with pathologic complete response after neoadjuvant therapy (ypCR) [15,16,17,18].
The survival outcomes of patients with stages 0 and II breast cancer after NCT were not significantly different (Figure 4). Because we combined ypT0N0M0 and ypTisN0M0 after NCT into yp stage 0, they were all considered ypCR. ypCR showed excellent survival outcomes in previous studies [16,17,19]. Moreover, a previous study reported that patients with ypCR experienced the best survival outcomes, and the recurrence rate and mortality increased significantly as the ypTNM stage increased [16]. The study results suggested that the survival outcomes of ypCR were not significantly different in the patients with in situ carcinoma without NCT [19]. Therefore, when patients have achieved complete pathologic remission after NCT, their outcomes may be similar to those with primary stage 0 breast cancer. No differences in survival outcomes were observed between yp and p stage II. Further investigations are warranted to evaluate the implication of stage II after NCT.
Figure 4. Changes of OS according to NCT in each prognostic stage; (A) stage 0, (B) stage I, (C) stage II, (D) stage III, (E) stage IV.
OS = overall survlval; NCT = neoadjuvant chemotherapy.
The current study had some limitations. In particular, approximately 30% patients had an unknown HER2 status in the registry because of a lack of HER2 overexpression assays prior to 2010. Hence, application of HER2 status to the prognostic staging system was limited. In addition, selection bias may exist due to the retrospective single-center nature of this study. However, the patient populations in this study received equivalent surgical procedures and pre- and postoperative treatment, which made the study populations homogeneous. When the 8th edition of the AJCC manual was published, the prognostic staging table had missing subgroups [1], and there were > 20 uncategorized combinations of T, N, grade, and hormonal receptor statuses. We integrated those patient groups into the prognostic staging system using a recent software to assist with the AJCC cancer staging.
This study demonstrates that the prognostic staging system of the 8th edition of the AJCC manual discriminates survival outcomes better than the anatomical staging system of the 7th edition of the AJCC manual. Staging migration from stage II in the anatomical staging system to stage I or III in the prognostic staging system was commonly observed. ypCR showed excellent survival outcomes and no statistically significant difference in survivals compared to in situ carcinomas. The new staging system appears to be a superior alternative to the conventional anatomic staging system.
Footnotes
Funding: This research was supported by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (2016R1D1A1B03934564), a Severance Surgeon's Alumni Research Grant (2016-01) and a faculty research grant of Yonsei University College of Medicine (6-2017-0072).
Conflict of Interest: The authors declare that they have no competing interests.
- Conceptualization: Kim EJ, Park HS.
- Data curation: Kim EJ, Park HS, Kim JY, Kim SI.
- Formal analysis: Kim EJ.
- Funding acquisition: Park HS.
- Investigation: Kim EJ, Park HS, Kim JY, Kim SI.
- Methodology: Park HS.
- Project administration: Kim EJ, Park HS.
- Resources: Park HS, Kim JY.
- Supervision: Park HS.
- Validation: Park HS, Kim SI, Cho YU, Park BW.
- Visualization: Kim EJ.
- Writing - original draft: Kim EJ.
- Writing - review & editing: Kim EJ.
SUPPLEMENTARY MATERIALS
OS of each staging system
RFS of each staging system
References
- 1.Weiss A, Chavez-MacGregor M, Lichtensztajn DY, Yi M, Tadros A, Hortobagyi GN, et al. Validation study of the American Joint Committee on Cancer eighth edition prognostic stage compared with the anatomic stage in breast cancer. JAMA Oncol. 2018;4:203–209. doi: 10.1001/jamaoncol.2017.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amin MB, Edge SB. AJCC Cancer Staging Manual. New York (NY): Springer; 2017. [Google Scholar]
- 3.Gospodarowicz MK, Brierley JD, Wittekind C. TNM Classification of Malignant Tumours. Chichester: Wiley; 2017. [Google Scholar]
- 4.Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The eighth edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67:93–99. doi: 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]
- 5.Harris LN, Ismaila N, McShane LM, Andre F, Collyar DE, Gonzalez-Angulo AM, et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34:1134–1150. doi: 10.1200/JCO.2015.65.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang M, Chen H, Wu K, Ding A, Zhang M, Zhang P. Evaluation of the prognostic stage in the 8th edition of the American Joint Committee on Cancer in locally advanced breast cancer: an analysis based on SEER 18 database. Breast. 2018;37:56–63. doi: 10.1016/j.breast.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 7.Wong RX, Wong FY, Lim J, Lian WX, Yap YS. Validation of the AJCC 8th prognostic system for breast cancer in an Asian healthcare setting. Breast. 2018;40:38–44. doi: 10.1016/j.breast.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Giuliano AE, Connolly JL, Edge SB, Mittendorf EA, Rugo HS, Solin LJ, et al. Breast cancer-major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:290–303. doi: 10.3322/caac.21393. [DOI] [PubMed] [Google Scholar]
- 9.Park S, Park BW, Kim TH, Jeon CW, Kang HS, Choi JE, et al. Lack of either estrogen or progesterone receptor expression is associated with poor survival outcome among luminal A breast cancer subtype. Ann Surg Oncol. 2013;20:1505–1513. doi: 10.1245/s10434-012-2772-x. [DOI] [PubMed] [Google Scholar]
- 10.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park S, Park HS, Koo JS, Yang WI, Kim SI, Park BW. Breast cancers presenting luminal B subtype features show higher discordant human epidermal growth factor receptor 2 results between immunohistochemistry and fluorescence in situ hybridization. Cancer. 2012;118:914–923. doi: 10.1002/cncr.26406. [DOI] [PubMed] [Google Scholar]
- 12.Ibis K, Ozkurt S, Kucucuk S, Yavuz E, Saip P. Comparison of pathological prognostic stage and anatomic stage groups according to the updated version of the American Joint Committee on Cancer (AJCC) breast cancer staging 8th edition. Med Sci Monit. 2018;24:3637–3643. doi: 10.12659/MSM.911022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu H, Wei W, Yi X, Xin L, Liu Y. A retrospective analysis of clinical utility of AJCC 8th edition cancer staging system for breast cancer. World J Oncol. 2017;8:71–75. doi: 10.14740/wjon1039e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdel-Rahman O. Validation of the 8th AJCC prognostic staging system for breast cancer in a population-based setting. Breast Cancer Res Treat. 2018;168:269–275. doi: 10.1007/s10549-017-4577-x. [DOI] [PubMed] [Google Scholar]
- 15.von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 16.Campbell JI, Yau C, Krass P, Moore D, Carey LA, Au A, et al. Comparison of residual cancer burden, American Joint Committee on Cancer staging and pathologic complete response in breast cancer after neoadjuvant chemotherapy: results from the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657) Breast Cancer Res Treat. 2017;165:181–191. doi: 10.1007/s10549-017-4303-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazouni C, Peintinger F, Wan-Kau S, Andre F, Gonzalez-Angulo AM, Symmans WF, et al. Residual ductal carcinoma in situ in patients with complete eradication of invasive breast cancer after neoadjuvant chemotherapy does not adversely affect patient outcome. J Clin Oncol. 2007;25:2650–2655. doi: 10.1200/JCO.2006.08.2271. [DOI] [PubMed] [Google Scholar]
- 18.Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 19.Jones RL, Lakhani SR, Ring AE, Ashley S, Walsh G, Smith IE. Pathological complete response and residual DCIS following neoadjuvant chemotherapy for breast carcinoma. Br J Cancer. 2006;94:358–362. doi: 10.1038/sj.bjc.6602950. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
OS of each staging system
RFS of each staging system