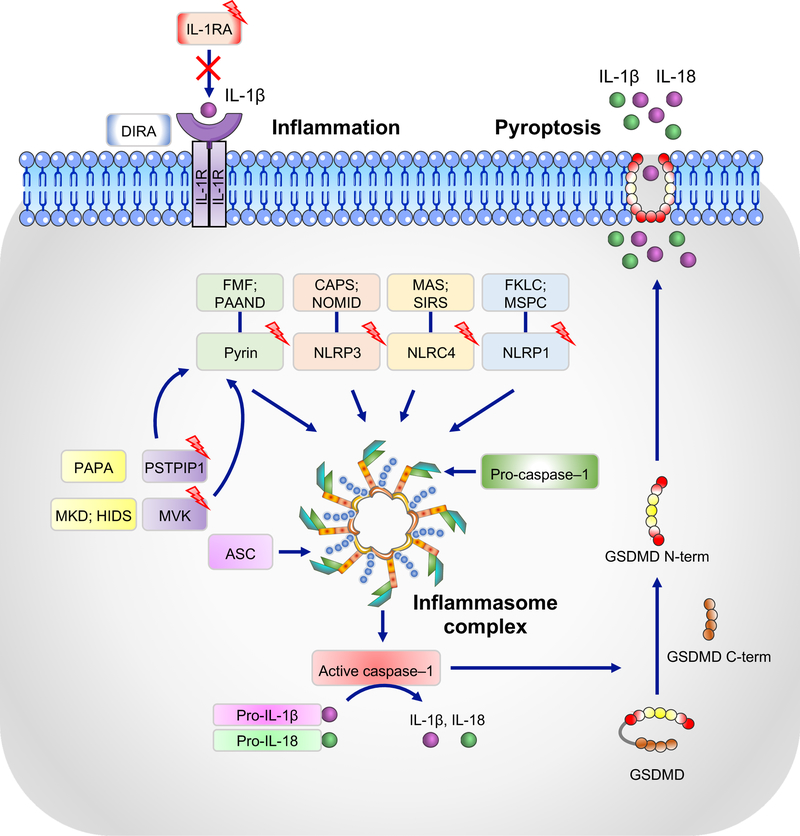
Figure 3. Inflammasomes and their association with autoinflammatory syndromes.
NLRP1, NLRP3, NLRC4, and pyrin form canonical inflammasomes with ASC and caspase-1 that process pro–IL-1β and pro–IL-18 into their active forms, IL-1β and IL-18. Boxes with lightening bolts denote proteins that have been reported to be mutated in the respective diseases. Gain-of-function mutations in these proteins lead to constitutively active inflammasomes. Diseases associated with corresponding mutations are: NLRP1, multiple self-healing palmoplantar carcinoma (MSPC) and familial keratosis lichenoides chronica (FKLC) [153]; NLRP3, cryopyrin-associated periodic syndrome (CAPS) and neonatal onset multisystem inflammatory disease (NOMID); NLRC4, macrophage activation syndrome (MAS) and systemic inflammatory response syndrome (SIRS); pyrin, familial Mediterranian fever (FMF) and pyrin-associated autoinflammation with neutrophilic dermatosis (PAAND). Mutations in mevalonate kinase (MVK) and proline-serine-threonine phosphatase-interacting protein 1 (PSTPIP1) associated with mevalonate kinase deficiency (MKD) and/or hyper IgD syndrome (HIDS) and purulent arthritis, pyoderma gangrenosum, and cystic acne (PAPA), respectively, affect the activity of the pyrin inflammasome through various mechanisms and lead to increased IL-1β secretion. Deficiency in the IL-1 receptor antagonist (IL-1RA), which is associated with deficiency of IL-1 receptor antagonist (DIRA), leads to unchecked IL-1 signaling. Activated caspase-1 can also cleave gasdermin D (GSDMD), and its released N-terminal domain (N-term) forms cytotoxic pores in the plasma membrane, thus inducing pyroptosis, an inflammatory form of cell death.