Abstract
Morphine is a potent analgesic opiate commonly used in treating pain, and it is also a substance of abuse and highly addictive. Hence, it is vital to discover the action sites of morphine in the brain to increase its efficacy of treatment. In the present study, we aimed at identifying comprehensive neuroanatomical locations that are sensitive to morphine in the adult zebrafish (Danio rerio). We performed in situ hybridization to localize the mu opioid receptor (oprm1) gene and to map the morphine sensitive brain areas using neuronal PAS domain-containing protein 4a (npas4a), an early gene marker. Real-time PCR was used to detect changes in mRNA levels of oprm1 and npas4a in control and acute morphine treated fish (2 mg/L; 20 min). Intense positive oprm1 signals were seen in the telencephalon, preoptic area, habenula, hypothalamic area and periventricular gray zone of the optic tectum. Acute morphine exposure significantly increased oprm1 and npas4a mRNA levels in the medial zone of dorsal telencephalon (Dm), ventral region of the ventral telencephalon (Vv), preoptic area, and in the hypothalamus but a decrease in oprm1 and npas4a signals in the dorsal habenula. This study provides a detailed map of oprm1 localization in the brain, which includes previously unreported oprm1 in the habenula of teleost. Presence of oprm1 in multiple brain sites implies multiple action targets of morphine and potential brain functions which could include reward, cognitive and negative emotions.
Keywords: opiates, mu opioid receptor, cfos, NPAS4a, teleosts
Introduction
Morphine is a well-documented analgesic drug used to treat severe pain. In addition to its analgesic effect, morphine also exerts its rewarding properties, which leads to opioid addiction. The multiple action sites of morphine in the brain decrease the effectiveness of morphine due to development of tolerance, physical dependence, and addiction.
Morphine can bind to μ (mu) opioid receptor (MOR), δ (delta) opioid receptor (DOR) and κ (kappa) opioid receptor (KOR). However, it primarily binds to MOR to exert its analgesic pharmacological properties (Corbett et al., 1993; Kieffer, 1999). In the brain of mammals, MOR are widely expressed in several areas including the periaqueductal gray, thalamus, cingulate cortex and the insula, which is involved in mediating pain signals (Kitchen et al., 1997; Le Merrer et al., 2009; Lutz and Kieffer, 2013; Cahill et al., 2014), while MOR in the ventral tegmental area (VTA) and the nucleus accumbens (NAc) are involved in opiate-induced reward (Contet et al., 2004). The reinforcement effect of morphine is triggered by activating dopamine release from the VTA and the NAc (Hyman and Malenka, 2001; Ballantyne and LaForge, 2007). Expression of MOR is also reported in the habenula—interpeduncular nucleus (IPN) pathway; suggesting the potential role of MOR in mediating the positive and negative effect of opioids, which needs to be further investigated (Gardon et al., 2014). The current therapeutic medications manage opioid relapse, yet overdose fatalities and recurrent cases of substance abuse are increasing (King et al., 2014; McLaughlin et al., 2017). Hence, identifying the comprehensive distribution of MOR is important to elucidate the target sites and understanding of brain regions that are potentially affected by morphine.
Various regulatory molecules and intracellular signaling pathway have been implicated in the regulation of MOR expression; therefore, along with the receptor expression profile, the neural activation can provide complementary details on the brain region that are sensitive to morphine. Several electrophysiological studies in mammalian species have demonstrated the neural activation of specific brain regions or cell types in response to morphine (Iwatsubo and Clouet, 1977; Nowycky et al., 1978; Matthews and German, 1984; Diana et al., 1999; Su et al., 2012; Bull et al., 2017). Mapping of neuronal activation sites in response to morphine has also been examined using neuronal activation markers, such as c-Fos, which is an immediate early gene and extracellular signal-regulated kinase (Chang et al., 1988; Hayward et al., 1990; Valjent et al., 2004). Although most neuronal activation markers are induced by a variety of stimuli, their expression is mainly induced as a result of molecular signaling for normal cellular function and survival and not solely due to the neural activation (Ramanan et al., 2005; Ramamoorthi et al., 2011). Recently, neuronal PAS domain protein 4 (Npas4) has been identified as a brain-enriched transcription factor, which is selectively coupled to neuronal activity and can provide accurate neural activation profile (Lin et al., 2008). Although MOR distributions have been demonstrated in several mammalian and in non-mammalian vertebrates, their expression pattern and neural activation sites upon opioid treatment are less studied.
The zebrafish (Danio rerio) possesses the MOR-homologous gene (oprm1, also known as ZFOR2) (Barrallo et al., 2000) with similar pharmacological characteristics to MOR in mammals (de Velasco et al., 2009). Although the distribution of the oprm1 gene and protein in the whole tissue has been demonstrated in larval zebrafish (Bretaud et al., 2007; Sanchez-Simon and Rodriguez, 2008; Arévalo et al., 2018), but their detailed expression patterns in the adult brain remains unreported. In the present study, we first examined the expression sites of the oprm1 gene in the brain of adult zebrafish using in situ hybridization. Next, to identify brain regions sensitive to morphine, we examined the effect of acute (20-min) morphine exposure on oprm1, cfos and npas4a gene expression in the brain by in situ hybridization and real-time PCR.
Materials and Methods
Animal and Housing
Sexually mature male (4–6 months, 0.5–1.0 g body weight), the RIKENWako (RW) wild-type zebrafish (Danio rerio; RRID: ZDB-GENO-030619-2), obtained from the National BioResource Center of Japan (www.shigen.nigac.jp/zebra/) were maintained in freshwater aquaria at 27 ± 0.5°C under a controlled natural photo-regimen (14/10 h, light/dark phase). The fish were fed with an Adult Zebrafish Diet (Zeigler, Gardners, PA, USA) twice daily. The fish were maintained, and all the experiments were carried out under the guideline of the Animal Ethics Committee of Monash University (ethics approval number: MARP/2017/049). However, this study is not pre-registered.
Acute Morphine Treatment
To reduce handling stress, fish were acclimatized for a week before the treatment. Fish were then assigned randomly to acute morphine treatment or control; no particular randomization method was performed to allocate the subjects in groups in this study. Fish were individually treated by immersion in a tank [sized 361 mm (L) × 218 mm (W) × 256 mm (D)] with water containing 2 mg/L morphine sulfate pentahydrate (Lipomed AG, Switzerland) for 20 min. The morphine treatment procedure was chosen based on a protocol previously reported (Stewart et al., 2011). As morphine is an analgesic, it does not cause any form of pain or suffering to the fish, and the doses used are those utilized in previous studies that do not cause toxicity (Magalhães et al., 2017; Chatigny et al., 2018). Therefore, no major effects from the treatments on fish were expected and only normal healthy fish were used in this study. After the treatment, the fish were anesthetized by immersion in water containing benzocaine (0.1 g benzocaine/200 mL, Sigma) and the brain was dissected for in situ hybridization and real-time PCR analysis. The same treatment protocol was employed for control samples, but they were immersed 20 min in water without morphine. In both the morphine and control group, the treatments were carried out simultaneously on separate immersion tanks from 1,400 to 1,600 h.
In situ Hybridisation of Zebrafish oprm1, cfos, and npas4a Genes
The sense and antisense digoxigenin (DIG) labeled-riboprobes for oprm1, cfos and npas4a were transcribed from a pGEM T-Easy vector (Promega, Madison, WI) containing 1,180, 438, and 737 bp fragments of zebrafish cDNA [GenBank accession numbers: NM_131707, NM_205569.1, and NM_001045321, respectively; the National Center for Biotechnology Information (NCBI, RRID: nif-0000-00139)]. DIG labeling was achieved using MAXIscript (Cat# AM1322M, Ambion, Austin, TX) and DIG RNA labeling mix (Cat# 11277073910, Roche Diagnostics, Mannheim, Germany) following the manufacturers' instructions. The brain samples were fixed in buffered 4% paraformaldehyde for 6 h at 4°C, cryoprotected in 20% sucrose solution, and embedded in Tissue Tek OCT compound (Sakura Finetechnical, Tokyo, Japan). The specificity of the probes were examined using sagittal sections (n = 2 for each gene). Coronal sections (n = 6 per group for each gene) were used to examine the detailed expression of the genes. Brain sections (14 μm thickness) were cut in a cryostat and thaw-mounted onto 3-aminopropylsilane (APS)-coated glass slides. DIG-in situ hybridization was performed as described previously (Ogawa et al., 2012). Briefly, the sections were permeabilised with 0.2 M HCl and then treated with proteinase K (1 μg/mL) for 15 min, and hybridized with DIG-labeled riboprobes (50 ng/mL) at 55°C overnight in a humidified chamber. Following hybridization, the sections were washed and blocked with 2% normal sheep serum. The DIG-labeled probes then detected with an alkaline phosphatase-conjugated anti-DIG antibody (Roche Cat# 11093274910, RRID: AB_514497, diluted 1:500). For the localization of oprm1, cfos, and npas4a probe expressions, the chromogenic reaction was achieved with 4-nitroblue tetrazolium chloride/5-bromo-4-chloro-3-indolyl-phosphate (NBT/BCIP, Roche Cat# 11681451001). To examine the effect of morphine on oprm1, the signals were amplified using Tyramide Signal Amplification (TSA) Plus Fluorescein kit (Perkin-Elmer, Wellesley, MA) according to the manufacturer's instructions. Preliminary evaluation showed no difference in localization and staining intensity of Dig-labeled cfos expressing cells. Therefore, in subsequent experiments npas4a and not c-fos was used as a neuronal activity marker.
Image Capturing, Cell Counting, and Statistical Analysis
The DIG-stained sections were cover-slipped, scanned, and the images were then captured with a Zeiss MIRAX Midi Slide scanning system (Cat# 000000-1496-488, Zeiss, Göttingen, Germany) at a resolution of 230 nm using a ×20 objective and processed with the Mirax Viewer Image Software (3DTech, Budapest, Hungary). To standardize sections with different background intensity, all the section were changed to gray mode using adobe illustrator software CS5.1. For the manual counting of the number of DIG-labeled npas4a expressing cells (control n = 6, acute morphine-treated n = 6), an average of 10 consecutive sections/region for each sample were used. Single blinding procedure was used to count the cell number. No sample calculation was performed to predetermine the sample size. However, the sample size in this study is comparable to that in the previous study on neuronal activity quantification in zebrafish (Lau et al., 2011). Numbers of cells expressing npas4a were counted in regions showing prominent changes including the dorsal and ventral telencephalon (155 mm2), anterior preoptic area (186 mm2), posterior preoptic area (49 mm2), habenula (85 mm2), and the hypothalamic region (160 mm2). Cell counting was not performed in periventricular gray zone of optic tectum (PGZ) due to the high density and compact nature of npas4a cells in this area. All cell counting data were analyzed using the IBM statistical package V22.0 (IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp). Independent t-test was used for comparison between control and treated groups. Data are expressed as mean ± SEM with the significance set at P < 0.05. While for the semi-quantitative analysis of oprm1 and npas4a mRNA, staining density was subjectively scored on a five-point scale as follows + + + (high), + + (moderate), + (low), and – (absent). Nomenclature for the zebrafish brain regions was adopted from (Wullimann et al., 1996; Rink and Wullimann, 2001; Mueller et al., 2004; Mueller and Guo, 2009; Liu et al., 2015).
Quantification of oprm1 and npas4a mRNA Levels in the Macrodissected Brain Regions Using Real-Time PCR
Gene expression levels of oprm1 and npas4a were examined by real-time PCR in both the control and acute morphine-treated group (n = 20; control group = 10, treated = 10). Using forceps and under the stereoscopic microscope, the brain was divided into six regions comprising (i) the olfactory bulb, (ii) telencephalon including the preoptic area and habenula, (iii) hypothalamic region, (iv) optic tectum, (v) cerebellum, and (vi) hind brain region as shown in Figure 3A. The total RNA was isolated from six brain regions using 100 μl of Trizol (Life Technologies, Gaithersburg, MD) reagent according to the manufacturer's instruction. The extracted total RNA was dissolved in 20 μl diethylpyrocarbonate-treated water (DEPC-MQ), and RNA concentration and purity were quantified using NanoDrop Spectrophotometer ND 1000 (Thermo Fisher Scientific). One microgram of total RNA was then reverse transcribed to cDNA using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems) in a 20-μl reaction mixture containing 1× RT buffer, 1× deoxynucleotide triphosphate mix, 1× RT random primers, 20 U of ribonuclease inhibitor, and 10 U of MultiScribe Reverse Transcriptase. The cDNA was then subjected to real-time PCR for oprm1, npas4a, and β-actin (actb1, reference gene) (GenBank accession no NM_131707, NM_001045321, and NM_131031). The primer sequences were as follows: oprm1; F: 5′-ATGGGACTGGTGGGAAACG-3′ and R: 5′-GCCAAGGAATCTGCTAGAGCAA-3′, npas4a; F: 5′-CCTGGGGCAACAACCTGA-3′ and R:5′-CTTCCACTCCCATCTTTGCGG-3′, and β-actin; F: 5′-AGAGCTATGAGCTGCCTGACG-3′ and R: 5′-CCGCAAGATTCCATACCCA-3′. The total 10 μl PCR reaction mixture contained 2× SensiFAST SYBR Hi-ROX Kit Mix (Bioline), 0.1 μM each of forward and reverse primers (Table 1), and 1 μl of sample cDNA, which was analyzed using an ABI PRISM 7500 Sequence Detection System (Applied Biosystems). DEPC-MQ was used as a negative control (non-template control). The reaction program consisted of 50°C for 2 min, 95°C for 10 min, and 40 cycles of 95°C for 15 s and 60°C for 1 min, followed by a dissociation stage. The threshold cycle (Ct) of each gene was determined and normalized to the β-actin mRNA levels. The data were then analyzed according to the relative gene expression calculated by 2−ΔΔCt. All data are presented as means ± SEM, and statistical analyses were performed using an independent t-test to observe comparisons between control and morphine-treated fish.
Table 1.
Expression of oprm1 mRNA in the brain of adult zebrafish.
| Brain regions | ISH staining distribution | Brain regions | ISH staining distribution | ||||
|---|---|---|---|---|---|---|---|
| OPRM1 | OPRM1 | ||||||
| Abbr | Control | Treated | Abbr | Control | Treated | ||
| Telencephalon | |||||||
| Olfactory Bulbs | OB | Synencephalon | |||||
| Lateral olfactory tract | LOT | + | + | Nuclues of MLF | NMLF | ++ | ++ |
| Medial olfactory tract | MOT | + | + | Periventricular gray zone of optic tectum | PGZ | +++ | +++ |
| Primary olfactory fiber layer | POF | – | – | Periventricular pretectal nucleus, dorsal part | PPd | + | + |
| Glomerular layer of olfactory bulb | GL | + | + | Periventricular pretectal nucleus, ventral part | PPv | ++ | +++ |
| External cellular layer of olfactory bulb | ECL | ++ | ++ | ||||
| Internal cellular layer of olfactory bulb | ICL | ++ | ++ | Mesencephalon | |||
| Dorsal telecephalic area | Tectum Opticum | ||||||
| Dorsal telecephalic area | D | ++ | ++ | Optic tectum | TeO | ++ | +++ |
| Dorsal zone of D | Dd | + | + | Torus longitudinalis | TL | +++ | +++ |
| Lateral zone of D | Dl | ++ | ++ | Commisura tecti | Ctec | – | – |
| Central zone of D | Dc | + | + | Torus semicircularis | |||
| Medial zone of D | Dm | ++ | +++ | Central nucleus of torus semicircularis | TSc | + | + |
| Posterior zone of D | Dp | + | + | Ventrolateral nucleus of torus semicircularis | TSvl | + | + |
| Nucleus taeniae | NT | + | + | Tegmentum | |||
| Dorsal tegmental nucleus | DTN | + | ++ | ||||
| Ventral telecephalic area | V | Nucleus of the lateral lemniscus | NLL | + | ++ | ||
| Central nucleus of V | Vc | – | – | Nucleus Interpeduncularis | Nin | ++ | ++ |
| Dorsal nucleus of V | Vd | ++ | ++ | Vascular lacuna of area postrema | Vas | + | + |
| Lateral nucleus of V | Vl | – | – | Superior reticulam formation | SRF | + | + |
| Ventral nucleus of V | Vv | +++ | +++ | ||||
| Supracommissural nucleus of V | Vs | ++ | ++ | Rhombencephalon | |||
| Postcommissural nucleus of V | Vp | ++ | ++ | Cerebellum | |||
| Entopeduncular nucleus, dorsal part | End | + | + | Eminentia granularis | EG | ++ | ++ |
| Entopeduncular nucleus, ventral part | ENv | + | + | Corpus cerebelli | Cce | ++ | ++ |
| Vavula cerebelli | |||||||
| medial | Vam | + | + | ||||
| Diencephalon | lateral | Val | + | + | |||
| Preoptic area | Commmsura cerebelli | Ccer | + | + | |||
| Diencephalic ventricle | DiV | – | – | Medulla oblongata | |||
| parvocellular preoptic nucleus, anterior part | PPa | +++ | +++ | Medial longitudinal fascicle | MLF | + | + |
| parvocellular preoptic nucleus, posterior part | PPp | +++ | +++ | Ventrolateral longitudinal fascicle | Nvmv | + | + |
| Suprachiasmatic nucleus | SC | + | + | Superior raphe nucleus | SR | ++ | ++ |
| Magnocellular preoptic nucleus | PM | + | + | Griseum centrale | GC | + | + |
| Epithalamus | |||||||
| Dorsal Habenula | dHb | ++ | + | ||||
| Ventral Habenula | vHb | + | + | ||||
| Dorsal Thalamus | |||||||
| Central posterior thalamic nucleus | CP | + | + | ||||
| Anterior thalamic nucleus | A | + | + | ||||
| Ventral Thalamus | |||||||
| Intermediate thalamic nucleus | I | + | + | ||||
| Ventrolateral thalamic nucleus | VL | + | + | ||||
| Ventromedial thalamic nucleus | VM | + | + | ||||
| Posterior tuberculum | |||||||
| Posterior tuberal nucleus | PTN | + | ++ | ||||
| Lateral preglomerular nucleus | PGl | + | ++ | ||||
| Medial preglomerular nucleus | PGm | + | ++ | ||||
| Torus lateralis | Tla | + | ++ | ||||
| Hypothalamus | |||||||
| Dorsal zone of periventricular hypothalamus | Hd | + | ++ | ||||
| Ventral zone of periventricular hypothalamus | Hv | + | ++ | ||||
| Anterior tuberal nucleus | ATN | + | ++ | ||||
| Lateral hypothalamic nucleus | LH | + | ++ | ||||
| Paraventricular organ | PVO | – | – | ||||
The chart is showing the summary of oprm1 mRNA expressing brain regions based on the data collected from alternate coronal sections labeled with oprm1 mRNA expressing cells.
Symbols indicate relative expression of oprm1 mRNA: + + + (high), ++ (moderate), + (low), and – (absent). Abbreviation represented by Abbr.
Results
DIG-in situ Localization of oprm1 mRNA in the Brain
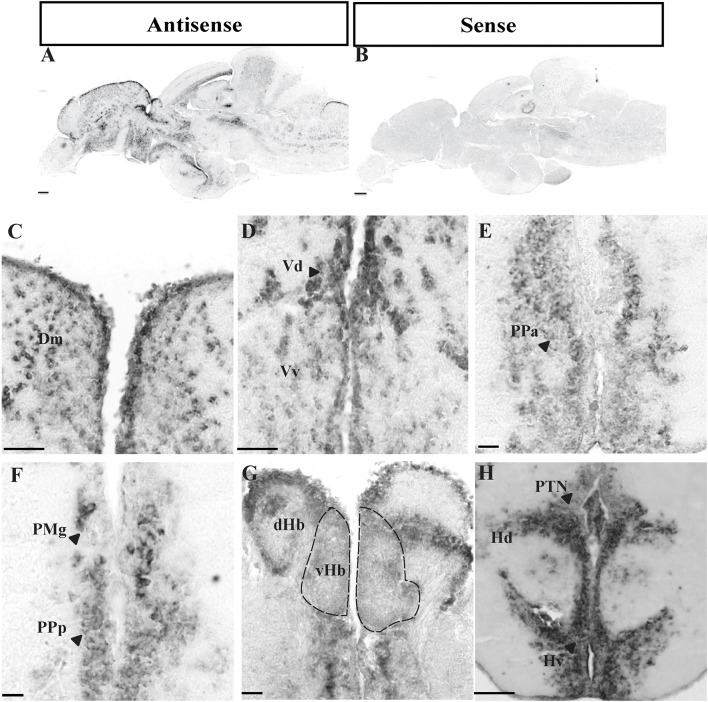
DIG-in situ hybridization showed expression of oprm1 throughout the zebrafish brain, which includes the telencephalon, diencephalon, mesencephalon, and the rhombencephalon, as summarized in Table 1 and illustrated in Figure 1. Sense probes did not show any signals (Figure 2) confirming the specificity of the antisense probes.
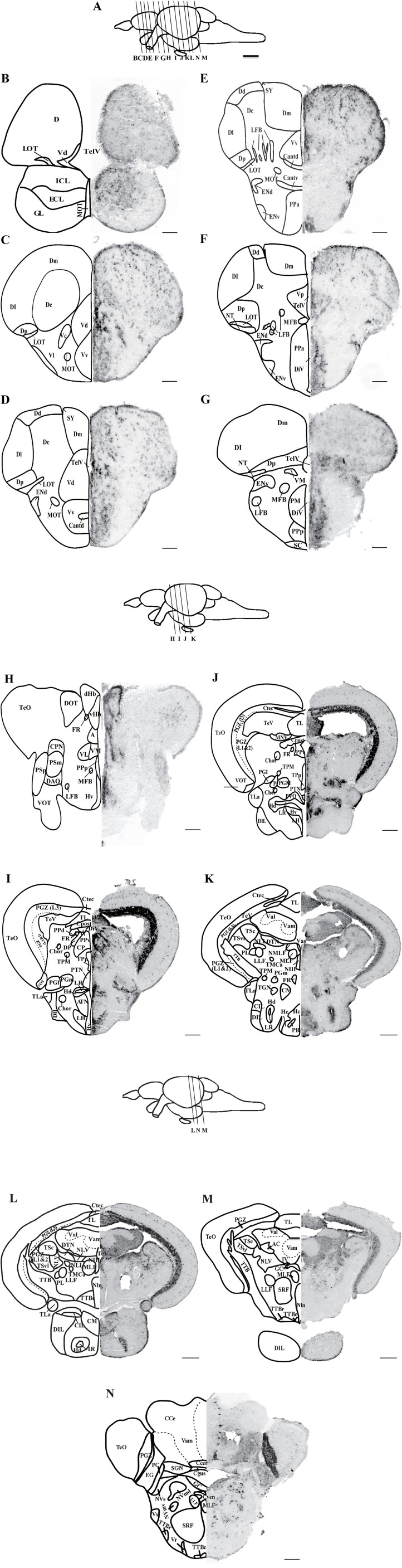
Figure 1.
Localization of oprm1 mRNA expression in the brain of zebrafish. Schematic coronal drawing of the brain of zebrafish showing the distribution of oprm1 mRNA expression. (A) Lines on a schematic sagittal drawing view of the zebrafish brain indicate levels of coronal sections, (B–N) coronal sections showing the distribution of oprm1 mRNA. n = 3, Scale bars: B–G (100 μm) and H–N (200 μm).
Figure 2.
Expression of oprm1 mRNA in the brain of zebrafish. Photomicrographs showing expression of oprm1 genes in sagittal (A,B) and coronal sections (C–H) of zebrafish brain. (A) Antisense probe for oprm1 gene showed positive signal and (B) Sense probes for oprm1 gene showed no signals. (C) Medial zone of dorsal telencephalic area, (D) Dorsal and Ventral nucleus of ventral telencephalic area, (E,F) Anterior and posterior part of parvocellular preoptic area, (G) Habenula area, and (H) Periventricular hypothalamic area. N = 3, Scale bars: A–B (100 μm) and C–H (50 μm).
Telencephalon
Although strong positively labeled oprm1 cells was observed in the internal and external cellular layer of olfactory bulb, relatively diffused distribution of oprm1 signals were also expressed (Figure 1B, Table 1). In the ventral telencephalon, oprm1 expressing cells lie in the dorsal and ventral nucleus of the ventral telencephalon (Figures 1C,D, 2D, Table 1). However, staining intensity in the ventral nucleus was weaker compared to the dorsal region of ventral telencephalon. Cells with strong signals were observed in almost all dorsal telencephalon subdivisions including the dorsal, lateral, medial, central, and posterior zone of dorsal telencephalic area (Figures 1B–G, Table 1). Strong positive signals were also observed in the dorsal and ventral part of the entopeduncular nucleus (Figures 1E–G, Table 1).
Diencephalon
Cells expressing oprm1 with intense staining were seen in the anterior, posterior parvocellular preoptic area and the magnocellular preoptic nucleus (Figures 1F,G, 2E,F, Table 1). In the epithalamus, cells expressing oprm1 lie in the dorsal and ventral habenula; however, the staining was quite diffuse (Figures 1H, 2G, Table 1). In the hypothalamic region, cells expressing oprm1 lie in the dorsal and ventral zone of the periventricular hypothalamus, lateral hypothalamic nucleus, and the posterior tuberal nucleus (Figures 1I, J, 2H, Table 1).
Mesencephalon
Cells expressing oprm1 lie in the optic tectum and the torus longitudinalis (Figure 1J). Strong signals were observed in the PGZ, however, within the PGZ nucleus and between sections containing the PGZ nucleus there was variations in signal intensity. In the tegmentum area, oprm1 expression was observed in the oculomotor nucleus and dorsal tegmental nucleus (Figure 1K).
Rhombencephalon
In the cerebellum, cells expressing oprm1 lie in several regions, such as the corpus cerebella, Eminentia granularis and few cells in lateral and medial division of valvula cerebelli (Figure 1N, Table 1). Some cells expressing oprm1 were also found in the medulla oblongata, such as the medial longitudinal fascicle and central gray (Figures 1M,N, Table 1).
Effect of Morphine on oprm1 Expression in the Brain of Zebrafish
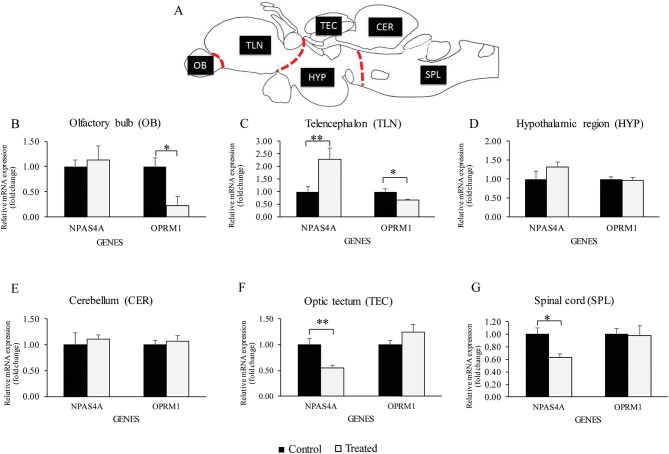
Using Real-time PCR significant down-regulation of oprm1 mRNA was seen in the olfactory bulb and telencephalon in morphine treated fish (Figures 3B,C). However, morphine had no effect on oprm1 mRNA in other brain regions (Figures 3D–G).
Figure 3.
Relative oprm1 and npas4a mRNA expression in the brain. (A–G) Graphs showing the relative gene expression (fold change) of oprm1 and npas4a mRNA expression after exposure to acute vehicle (Control) and morphine via water immersion (Treated) across several brain region. (A) Schematic sagittal drawing of macro-dissected brain region, (B) olfactory bulb region, (C) Telencephalon region, (D) Hypothalamic region, (E) Cerebellum, (F) Optic tectum, (G) Spinal cord region. Data are presented as mean ± SEM. Independent t-test comparisons between control and morphine-treated fish. *P < 0.05; **P < 0.01 vs. controls (n = 6).
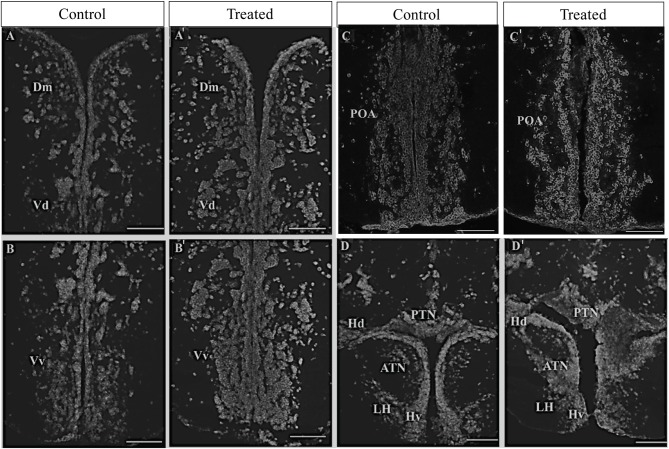
The effect of morphine on the oprm1 gene expression was further morphologically assessed via in situ hybridization (ISH). In morphine-exposed fish, ISH showed high signal intensity of oprm1 in the medial zone of dorsal telencephalon (Dm) and in the dorsal and ventral region of the ventral telencephalon (Vv and Vd) as compared to controls [Figure 4A (control), Figure 4A′ (morphine treated), Figures 4B,B′]. Similarly, in the posterior part of the parvocellular preoptic nucleus and periventricular hypothalamic region, higher signal intensity of oprm1 was observed in morphine-treated fish as compared to control fish (Figures 4C,C′,D,D′).
Figure 4.
Effect of morphine on oprm1 mRNA expression in the brain of zebrafish. Fish were individually immersed in morphine 2 mg/L (treated) or water (control) for 20 min and then the brain samples were collected. (A–D′) Photomicrographs showing expression of oprm1 genes. (A; control and A′; morphine treated) Medial zone of the dorsal telencephalic area and dorsal nucleus of the ventral telencephalic area, (B,B′) ventral nucleus of the ventral telencephalic area, (C,C′) posterior part of preoptic area, and (D,D′) ventral periventricular hypothalamus. N = 6 each group. Scale bars: (A–D) and (A′-D′) (100 μm).
Effect of Morphine on Npas4a Expression in the Brain of Zebrafish
Real-time PCR showed a significant increase in npas4a mRNA levels in the telencephalon (Figure 3C), but a decrease in the optic tectum and spinal cord in morphine treated fish (Figures 3F,G). There was no effect of morphine treatment on npas4a mRNA levels in other brain regions (Figures 3B,D,E).
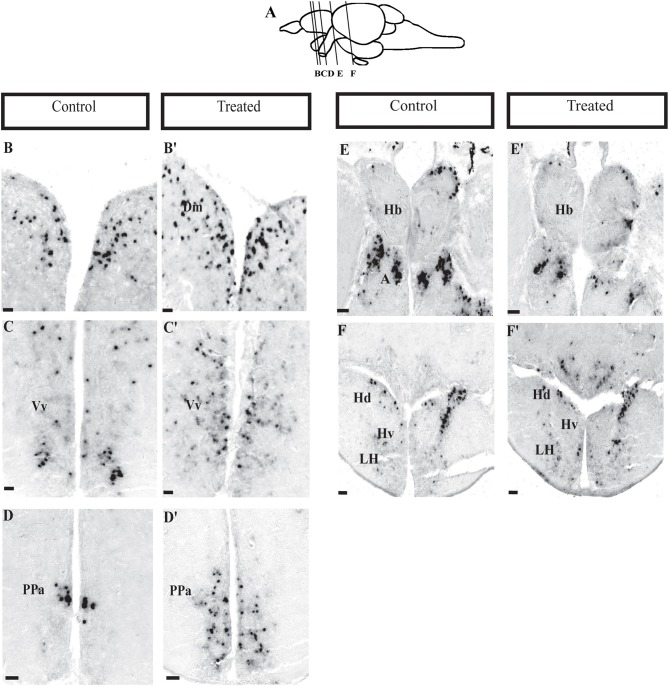
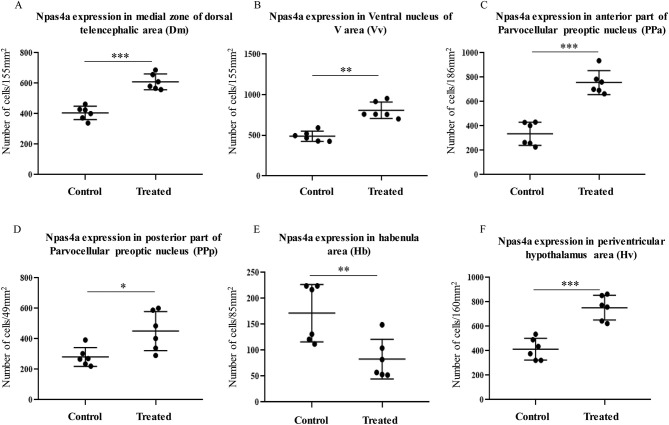
In situ hybridization, showed a significant changes in npas4a expression in several brain areas (Figure 5A). Increase in npas4a signal intensity were observed in the medial zone of dorsal and ventral nucleus of telencephalon, preoptic area and hypothalamus [Figures 5B–D,F (control), Figures 5B′-D′,F′ (morphine treated)], but a decrease in the dorsal habenula and in the anterior thalamic nucleus in morphine treated fish (Figure 5E). npas4a expressing cell numbers were significantly increase in the medial region of the dorsal telencephalon (Figure 6A), ventral region of the ventral telencephalon (Figure 6B), anterior and posterior part of the parvocellular preoptic nucleus (Figures 6C,D), and the periventricular hypothalamus area (Figure 6F) but cell numbers were significantly decreased in the dorsal habenula (Figure 6E).
Figure 5.
Expression of npas4a gene in the brain of zebrafish. Photomicrographs showing the mRNA expression of neural activity marker, npas4. (A) Lines on the schematic sagittal drawing view of the zebrafish brain indicate levels of coronal sections (B; control and B′; morphine treated) medial zone of dorsal telencephalic area, (C,C′) ventral nucleus of ventral telencephalic area, (D,D′) preoptic area, (E,E′) habenula, and (F,F′) ventral periventricular hypothalamus. N = 6 each group. Scale bars: (B–F) and (B′-F′) (100 μm).
Figure 6.
Number of npas4 mRNA expressing cells in control and morphine-treated fish. (A–F) Graphs showing the difference in npas4a mRNA expression via cell counting after exposure to acute vehicle (control) and morphine via water immersion. (A) Medial zone of the dorsal telencephalic area, (B) ventral nucleus of the ventral telencephalic area, (C,D) anterior and posterior part of parvocellular preoptic area, (E) habenula, and (F) ventral periventricular hypothalamus. Data are presented as mean ± SEM. Independent t-test comparisons between control and morphine-treated fish. *P < 0.05; **P < 0.01, ***P < 0.001 vs. controls (n = 6).
Discussion
In the present study, we investigated the distribution of cells expressing oprm1 mRNA in the brain of adult zebrafish. The ISH staining demonstrated wider distribution of oprm1 mRNA expression throughout the brain, which is similar to oprm1 expression that were previously demonstrated in the larval zebrafish brain using whole-mount in situ hybridization and a MOR antibody generated specific to zebrafish (Sanchez-Simon and Rodriguez, 2008; Arévalo et al., 2018). Although the distribution of MOR has been reported in the brain of several teleost species (Chadzinska et al., 2009; Stevens, 2009), this report is the first detailed mapping of MOR in the adult brain of a teleost, the zebrafish.
Previous studies in zebrafish larvae using whole-mount ISH have shown positive MOR signals and also immunoreactive cells in the telencephalon region (Sanchez-Simon and Rodriguez, 2008; Arévalo et al., 2018), likewise, in this study, we observed intense oprm1 positive signals in the dorsal and ventral telencephalon. In fish (rainbow trout, lamprey, eel, and coho salmon) (Bird et al., 1988; Ebbesson et al., 1996), and in birds (Reiner et al., 1989) MOR binding sites have been reported in the telencephalon. Similarly, in mammals MOR signals have been observed in telencephalic nuclei, which include nucleus accumbens, striatum, amygdala, and the hippocampus (Delfs et al., 1994; Mansour et al., 1994). These studies suggest the distribution of MOR in the telencephalon is well-conserved across vertebrate species, which could be a center for reward (Charbogne et al., 2017; Ben Hamida et al., 2019).
Positive oprm1 signal have been observed in the diencephalon of larvae zebrafish (Sanchez-Simon and Rodriguez, 2008), anterior preoptic area of rainbow trout (Bird et al., 1988) and medial preoptic area of rodents (Kaufman et al., 1995; Gulledge et al., 2000). Similarly, the present study shows a wide distribution of intense oprm1 signals in the anterior and posterior preoptic area. Interestingly, we observed oprm1 expression in the dorsal habenula similar to that in the medial habenula of rodents (Mansour et al., 1987, 1994; Gardon et al., 2014). There are no reports of MOR binding sites or MOR expression in the habenula of other non-mammalian vertebrates, including fish (Bird et al., 1988). The lack of MOR in the habenula of non-mammalian vertebrates could be due to low expression of the receptor, which could not be detected by ISH and radio-ligand binding assay, it could be an oversight or a complete absence of the receptor. MOR in the habenula has been implicated in mediating analgesia, aversive and reward functions in rodents. Since the mammalian medial habenula is homologous to the dorsal habenula in the zebrafish, this function of MOR could be conserved.
The current study also showed intense oprm1 positive signals in the optic tectum in particular in the periventricular gray zone of optic tectum (PGZ). In the optic tectum, immunoreactive cells have been reported in zebrafish larvae (Sanchez-Simon and Rodriguez, 2008), and MOR binding sites in the rainbow trout (Bird et al., 1988). In rodents, moderate expression of MOR has been reported in the superior colliculus, which is referred to as the optic tectum in non-mammalian vertebrates. Since the optic tectum is the site of neurogenesis in teleost (D'Angelo et al., 2014; Cacialli et al., 2016) and is involved in visual sensory functions, it is possible that MOR might be related to these functions.
In the present study, npas4a induced neuronal activity was seen within 20 min of morphine exposure. Therefore, in the zebrafish npas4a compared to c-fos, which takes 30–90 min (Lau et al., 2011; Sun and Lin, 2016) is probably a more reliable neuronal activity marker to map morphine sensitive brain regions. Interestingly, in our study, a single exposure to morphine was sufficient to activate npas4a expression in the telencephalic and diencephalic neuronal population. However, in rodents multiple and not single dose (subthreshold stimulus) of psychostimulant (Guo et al., 2012), except a single acute injection of cocaine are needed to activate npas4 (Ye et al., 2016). Morphine has poor lipid solubility (Newby et al., 2009); however, for adult zebrafish a single immersion in morphine solution for 30 min increased brain morphine concentration (Lau et al., 2006). Furthermore, adult zebrafish immersed in a solution containing butorphanol, a molecule chemically similar to morphine, exhibit significant analgesic effect (Schroeder and Sneddon, 2017), while zebrafish larvae immersed in a solution of morphine exhibit noxious stimulus (Lopez-Luna et al., 2017). In addition, blocking MOR using naloxone (MOR antagonist) reduces place preference behavior to morphine in both adult and larval zebrafish (Lau et al., 2006; Bretaud et al., 2007). These studies suggest that sufficient amounts of morphine reach all brain structures and provide evidence of selective binding of morphine to activate oprm1 in the zebrafish.
Following acute morphine exposure, oprm1 mRNA levels in macro-dissected olfactory bulb and telencephalon were significantly down-regulated, while ISH semi-qualitative signal analysis showed an increase in oprm1 in the telencephalon. On the other hand, npas4a mRNA expression and npas4a expressing cell numbers were increased in the telencephalon. Similarly, in rats, acute morphine treatment increases MOR density and neuronal activity in the forebrain especially in the nucleus accumbens (Haberstock-Debic et al., 2003), which is homologous to the dorsal nucleus of ventral telencephalon region (Vd) in teleost (O'Connell and Hofmann, 2011). The discrepancies between in situ hybridization and RT-PCR results may be due to limitations of the two techniques. Results obtained with the RT-PCR are from dissected small brain regions with a risk of dissection error and hence high variability in results. On the other hand, in situ hybridization is a sensitive method that allows detail anatomical localization, but the analysis of signal intensity is semi-quantitative.
Morphine treatment did not affect oprm1 and npas4a mRNA levels in the diencephalon but an increase in oprm1 and npas4a ISH signal and npas4a expressing cells were seen in the preoptic and hypothalamic area. Similarly, in female rats, oprm1 mRNA expression was increased in the preoptic-hypothalamic area upon morphine treatment (Petersen and LaFlamme, 1997; Šlamberová et al., 2005). The presence and upregulation of oprm1 gene together with npas4a expression in the preoptic-hypothalamic nuclei suggest the possibility of morphine interaction with the neuroendocrine and the monoamine systems in this brain region (Yu et al., 1991; Bellipanni et al., 2002; Prasad et al., 2015). In the habenula, morphine induced downregulation of oprm1 and npas4a expression. Although there are no reports on the regulation of MOR in the habenula of rodents, a reduced neuronal activity was reported in the previous study (Hashimoto et al., 2009); which is associated with anti-nociceptive effect and could potentially be related to many of habenula functions, such as negative emotions.
In the hindbrain, acute morphine treatment caused no change in oprm1 mRNA levels. However, more specific ISH staining showed an increase in oprm1 signal in the optic tectum area, torus longitudinalis and tegmental area. Meanwhile, acute morphine treatment down-regulates npas4a mRNA levels in the optic tectum and spinal cord but induction of npas4a was seen in the ventral entopeduncular nucleus, interpeduncular nucleus and Corpus cerebelli in the cerebellum, where very low expression of oprm1 was seen. Expression of npas4 function to promote a reduction in overall circuit activity through the late response genes (Spiegel et al., 2014). In rodents, morphine treatment could down-regulate glutamate transporter in the hindbrain and spinal cord (Mao et al., 2002). This suggests that several neuronal groups in the hindbrain might be regulated by morphine through indirect pathways. It should be noted that the activation or inhibition of opioid receptor signaling is associated with the different G protein interactions. Previous studies showed that the activation of the G-coupled protein receptor has led to decrease of neurotransmitter release or membrane potential hyperpolarization, and indirectly mediate morphine action (Mazei-Robison and Nestler, 2012; Cachope and Pereda, 2015). Thus, further analysis for G protein signaling pathways are necessary to interpret specific activation or inhibition of npas4a-expressing cells.
Summary
In the present study, we found specific cell populations that are sensitive to morphine. The wide distribution of oprm1 gene, as well as morphine-dependent expression of npas4a in the brain, indicate that morphine can act on multiple brain sites and neural circuits, which implies that morphine can potentially influence a variety of brain functions including reward, cognitive, and aversive functions in fish.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The animal study was reviewed and approved by Animal Ethics Committee of Monash University (ethics approval number: MARP/2017/049).
Author Contributions
MS, SO, and IP designed the research. MS performed the research and wrote the paper. SO and MS analyzed the data. SO and IP edited the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Monash University Malaysia for the Graduate Merit Research Scholarship to MS and for providing neuro-imaging facilities that enable to complete the research project.
Glossary
Abbreviations
- OPRM1
opioid receptor mu 1
- NPAS4a
neuronal PAS domain protein 4a
- MOR
mu opioid receptor
- VTA
ventral tegmental area
- NAc
nucleus accumbens.
Footnotes
Funding. This work was supported by the Malaysian Ministry of Higher Education, FRGS/1/2013/SKK01/MUSM/03/02 and FRGS/1/2014/ST03/MUSM/02/1 (to IP and SO).
References
- Arévalo J. C., Hernández-Jiménez E., Jiménez-González A., Torres-Valle M., Iwasaki R. S., López-Bellido R., et al. (2018). Generation and characterization of antibodies against opioid receptors from zebrafish. Int. J. Mol. Sci. 19:14. 10.3390/ijms19010014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballantyne J. C., LaForge K. S. (2007). Opioid dependence and addiction during opioid treatment of chronic pain. Pain 129, 235–255. 10.1016/j.pain.2007.03.028 [DOI] [PubMed] [Google Scholar]
- Barrallo A., Gonzalez-Sarmiento R., Alvar F., Rodriguez R. E. (2000). ZFOR2, a new opioid receptor-like gene from the teleost zebrafish (Danio rerio). Brain Res. Mol. Brain Res. 84, 1–6. 10.1016/S0169-328X(00)00152-2 [DOI] [PubMed] [Google Scholar]
- Bellipanni G., Rink E., Bally-Cuif L. (2002). Cloning of two tryptophan hydroxylase genes expressed in the diencephalon of the developing zebrafish brain. Mech. Dev. 119, S215–S220. 10.1016/S0925-4773(03)00119-9 [DOI] [PubMed] [Google Scholar]
- Ben Hamida S., Boulos L.-J., McNicholas M., Charbogne P., Kieffer B. L. (2019). Mu opioid receptors in GABAergic neurons of the forebrain promote alcohol reward and drinking. Addict. Biol. 24, 28–39. 10.1111/adb.12576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird D. J., Jackson M., Baker B. I., Buckingham J. C. (1988). Opioid binding sites in the fish brain: an autoradiographic study. Gen. Compar. Endocrinol. 70, 49–62. 10.1016/0016-6480(88)90093-7 [DOI] [PubMed] [Google Scholar]
- Bretaud S., Li Q., Lockwood B. L., Kobayashi K., Lin E., Guo S. (2007). A choice behavior for morphine reveals experience-dependent drug preference and underlying neural substrates in developing larval zebrafish. Neuroscience 146, 1109–1116. 10.1016/j.neuroscience.2006.12.073 [DOI] [PubMed] [Google Scholar]
- Bull F. A., Baptista-Hon D. T., Lambert J. J., Walwyn W., Hales T. G. (2017). Morphine activation of mu opioid receptors causes disinhibition of neurons in the ventral tegmental area mediated by β-arrestin2 and c-Src. Sci. Rep. 7:9969. 10.1038/s41598-017-10360-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachope R., Pereda A. E. (2015). Opioids potentiate electrical transmission at mixed synapses on the Mauthner cell. J. Neurophysiol. 114, 689–697. 10.1152/jn.00165.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacialli P., Gueguen M.-M., Coumailleau P., D'Angelo L., Kah O., Lucini C., et al. (2016). BDNF expression in larval and adult zebrafish brain: distribution and cell identification. PLoS ONE 11:e0158057. 10.1371/journal.pone.0158057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill C. M., Taylor A. M. W., Cook C., Ong E., Morón J. A., Evans C. J. (2014). Does the kappa opioid receptor system contribute to pain aversion? Front. Pharmacol. 5:253. 10.3389/fphar.2014.00253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadzinska M., Hermsen T., Savelkoul H. F. J., Verburg-van Kemenade B. M. L. (2009). Cloning of opioid receptors in common carp (Cyprinus carpio L.) and their involvement in regulation of stress and immune response. Brain Behav. Immun. 23, 257–266. 10.1016/j.bbi.2008.10.003 [DOI] [PubMed] [Google Scholar]
- Chang S. L., Squinto S. P., Harlan R. E. (1988). Morphine activation of c-fos expression in rat brain. Biochem. Biophys. Res. Commun. 157, 698–704. 10.1016/S0006-291X(88)80306-1 [DOI] [PubMed] [Google Scholar]
- Charbogne P., Gardon O., Martín-García E., et al. (2017). Mu opioid receptors in gamma-aminobutyric acidergic forebrain neurons moderate motivation for heroin and palatable food. Biol. Psychiatry 81, 778–788. 10.1016/j.biopsych.2016.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatigny F., Creighton C. M., Stevens E. D. (2018). Updated review of fish analgesia. J. Am. Assoc. Lab. Anim. Sci. 57, 5–12. [PMC free article] [PubMed] [Google Scholar]
- Contet C., Kieffer B. L., Befort K. (2004). Mu opioid receptor: a gateway to drug addiction. Curr. Opin. Neurobiol. 14, 370–378. 10.1016/j.conb.2004.05.005 [DOI] [PubMed] [Google Scholar]
- Corbett A. D., Paterson S. J., Kosterlitz H. W. (1993). Selectivity of ligands for opioid receptors, in Opioids, eds Herz A., Akil H., Simon E. J. (Berlin; Heidelberg: Springer Berlin Heidelberg; ), 645–679. 10.1007/978-3-642-77460-7_26 [DOI] [Google Scholar]
- D'Angelo L., De Girolamo P., Lucini C., Terzibasi E. T., Baumgart M., Castaldo L., et al. (2014). Brain-derived neurotrophic factor: mRNA expression and protein distribution in the brain of the teleost Nothobranchius furzeri. J. Comp. Neurol. 522, 1004–1030. 10.1002/cne.23457 [DOI] [PubMed] [Google Scholar]
- de Velasco E. M. F., Law P. Y., Rodriguez R. E. (2009). Mu opioid receptor from the zebrafish exhibits functional characteristics as those of mammalian mu opioid receptor. Zebrafish 6, 259–268. 10.1089/zeb.2009.0594 [DOI] [PubMed] [Google Scholar]
- Delfs J. M., Kong H., Mestek A., Chen Y., Yu L., Reisine T., et al. (1994). Expression of mu opioid receptor mRNA in rat brain: an in situ hybridization study at the single cell level. J. Comp. Neurol. 345, 46–68. 10.1002/cne.903450104 [DOI] [PubMed] [Google Scholar]
- Diana M., Muntoni A. L., Pistis M., Melis M., Gessa G. L. (1999). Lasting reduction in mesolimbic dopamine neuronal activity after morphine withdrawal. Eur. J. Neurosci. 11, 1037–1041. 10.1046/j.1460-9568.1999.00488.x [DOI] [PubMed] [Google Scholar]
- Ebbesson L. O. E., Deviche P., Ebbesson S. O. E. (1996). Distribution and changes in μ- and κ-opiate receptors during the midlife neurodevelopmental period of coho salmon, Oncorhynchus kisutch. J. Comp. Neurol. 366, 448–464. [DOI] [PubMed] [Google Scholar]
- Gardon O., Faget L., Chu Sin Chung P., Matifas A., Massotte D., Kieffer B. L. (2014). Expression of mu opioid receptor in dorsal diencephalic conduction system: new insights for the medial habenula. Neuroscience 277, 595–609. 10.1016/j.neuroscience.2014.07.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulledge C. C., Mann P. E., Bridges R. S., Bialos M., Hammer R. P. (2000). Expression of μ-opioid receptor mRNA in the medial preoptic area of juvenile rats. Brain Res. Dev. Brain Res. 119, 269–276. 10.1016/S0165-3806(99)00184-4 [DOI] [PubMed] [Google Scholar]
- Guo M.-L., Xue B., Jin D.-Z., Liu Z.-G., Fibuch E. E., Mao L.-M., et al. (2012). Upregulation of Npas4 protein expression by chronic administration of amphetamine in rat nucleus accumbens in vivo. Neurosci. Lett. 528, 210–214. 10.1016/j.neulet.2012.07.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberstock-Debic H., Wein M., Barrot M., et al. (2003). Morphine acutely regulates opioid receptor trafficking selectively in dendrites of nucleus accumbens neurons. J. Neurosci. 23, 4324–4332. 10.1523/JNEUROSCI.23-10-04324.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K., Amano T., Sakai N., Suzuki T., Narita M. (2009). Cell-dependent physiological synaptic action of morphine in the rat habenular nucleus: morphine both inhibits and facilitates excitatory synaptic transmission. Neurosci. Lett. 451, 270–273. 10.1016/j.neulet.2009.01.009 [DOI] [PubMed] [Google Scholar]
- Hayward M. D., Duman R. S., Nestler E. J. (1990). Induction of the c-fos proto-oncogene during opiate withdrawal in the locus coeruleus and other regions of rat brain. Brain Res. 525, 256–266. 10.1016/0006-8993(90)90872-9 [DOI] [PubMed] [Google Scholar]
- Hyman S. E., Malenka R. C. (2001). Addiction and the brain: the neurobiology of compulsion and its persistence. Nat. Rev. Neurosci. 2, 695–703. 10.1038/35094560 [DOI] [PubMed] [Google Scholar]
- Iwatsubo K., Clouet D. H. (1977). Effects of morphine and haloperidol on the electrical activity of rat nigrostriatal neurons. J. Pharmacol. Exp. Ther. 202, 429–436. [PubMed] [Google Scholar]
- Kaufman D. L., Keith D. E., Anton B., et al. (1995). Characterization of the murine μ opioid receptor gene. J. Biol. Chem. 270, 15877–15883. 10.1074/jbc.270.26.15877 [DOI] [PubMed] [Google Scholar]
- Kieffer B. L. (1999). Opioids: first lessons from knockout mice. Trends Pharmacol. Sci. 20, 19–26. 10.1016/S0165-6147(98)01279-6 [DOI] [PubMed] [Google Scholar]
- King N. B., Fraser V., Boikos C., Richardson R., Harper S. (2014). Determinants of increased opioid-related mortality in the United States and Canada, 1990–2013: a systematic review. Am. J. Public Health 104, e32–e42. 10.2105/AJPH.2014.301966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchen I., Slowe S. J., Matthes H. W. D., Kieffer B. (1997). Quantitative autoradiographic mapping of μ-, δ- and κ-opioid receptors in knockout mice lacking the μ-opioid receptor gene. Brain Res. 778, 73–88. 10.1016/S0006-8993(97)00988-8 [DOI] [PubMed] [Google Scholar]
- Lau B., Bretaud S., Huang Y., Lin E., Guo S. (2006). Dissociation of food and opiate preference by a genetic mutation in zebrafish. Genes Brain Behav. 5, 497–505. 10.1111/j.1601-183X.2005.00185.x [DOI] [PubMed] [Google Scholar]
- Lau B. Y. B., Mathur P., Gould G. G., Guo S. (2011). Identification of a brain center whose activity discriminates a choice behavior in zebrafish. Proc. Natl. Acad. Sci. U.S.A. 108, 2581–2586. 10.1073/pnas.1018275108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Merrer J., Becker J. A. J., Befort K., Kieffer B. L. (2009). Reward processing by the opioid system in the brain. Physiol. Rev. 89, 1379–1412. 10.1152/physrev.00005.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Bloodgood B. L., Hauser J. L., Lapan A. D., Koon A. C., Kim T.-K., et al. (2008). Activity-dependent regulation of inhibitory synapse development by Npas4. Nature 455, 1198–1204. 10.1038/nature07319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Bhattarai S., Wang N., Sochacka-Marlowe A. (2015). Differential expression of protocadherin-19, protocadherin-17, and cadherin-6 in adult zebrafish brain. J. Comp. Neurol. 523, 1419–1442. 10.1002/cne.23746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Luna J., Al-Jubouri Q., Al-Nuaimy W., Sneddon L. U. (2017). Reduction in activity by noxious chemical stimulation is ameliorated by immersion in analgesic drugs in zebrafish. J. Exp. Biol. 220, 1451–1458. 10.1242/jeb.146969 [DOI] [PubMed] [Google Scholar]
- Lutz P.-E., Kieffer B. L. (2013). Opioid receptors: distinct roles in mood disorders. Trends Neurosci. 36, 195–206. 10.1016/j.tins.2012.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhães F. E. A., de Sousa C. Á. P. B., Santos S. A. A. R., et al. (2017). Adult zebrafish (Danio rerio): an alternative behavioral model of formalin-induced nociception. Zebrafish 14, 422–429. 10.1089/zeb.2017.1436 [DOI] [PubMed] [Google Scholar]
- Mansour A., Fox C. A., Burke S., Meng F., Thompson R. C., Akil H., et al. (1994). Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J. Comp. Neurol. 350, 412–438. 10.1002/cne.903500307 [DOI] [PubMed] [Google Scholar]
- Mansour A., Khachaturian H., Lewis M. E., Akil H., Watson S. J. (1987). Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. J. Neurosci. 7, 2445–2464. [PMC free article] [PubMed] [Google Scholar]
- Mao J., Sung B., Ji R.-R., Lim G. (2002). Chronic morphine induces downregulation of spinal glutamate transporters: implications in morphine tolerance and abnormal pain sensitivity. J. Neurosci. 22, 8312–8323. 10.1523/JNEUROSCI.22-18-08312.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews R., German D. (1984). Electrophysiological evidence for excitation of rat ventral tegmental area dopamine neurons by morphine. Neuroscience 11, 617–625. 10.1016/0306-4522(84)90048-4 [DOI] [PubMed] [Google Scholar]
- Mazei-Robison M. S., Nestler E. J. (2012). Opiate-induced molecular and cellular plasticity of ventral tegmental area and locus coeruleus catecholamine neurons. Cold Spring Harb. Perspect. Med. 2:a012070. 10.1101/cshperspect.a012070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin I., Dani J. A., De Biasi M. (2017). The medial habenula and interpeduncular nucleus circuitry is critical in addiction, anxiety, and mood regulation. J. Neurochem. 142, 130–143. 10.1111/jnc.14008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller T., Guo S. (2009). The distribution of GAD67-mRNA in the adult zebrafish (teleost) forebrain reveals a prosomeric pattern and suggests previously unidentified homologies to tetrapods. J. Comp. Neurol. 516, 553–568. 10.1002/cne.22122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller T., Vernier P., Wullimann M. F. (2004). The adult central nervous cholinergic system of a neurogenetic model animal, the zebrafish Danio rerio. Brain Res. 1011, 156–169. 10.1016/j.brainres.2004.02.073 [DOI] [PubMed] [Google Scholar]
- Newby N. C., Wilkie M. P., Stevens E. D. (2009). Morphine uptake, disposition, and analgesic efficacy in the common goldfish (Carassius auratus). Can. J. Zool. 87, 388–399. 10.1139/Z09-023 [DOI] [Google Scholar]
- Nowycky M. C., Walters J. R., Roth R. H. (1978). Dopaminergic neurons: effect of acute and chronic morphine administration on single cell activity and transmitter metabolism. J. Neural Trans. 42, 99–116. 10.1007/BF01675349 [DOI] [PubMed] [Google Scholar]
- O'Connell L. A., Hofmann H. A. (2011). The Vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J. Comp. Neurol. 519, 3599–3639. 10.1002/cne.22735 [DOI] [PubMed] [Google Scholar]
- Ogawa S., Ramadasan P. N., Goschorska M., Anantharajah A., We Ng K., Parhar I. S. (2012). Cloning and expression of tachykinins and their association with kisspeptins in the brains of zebrafish. J. Comp. Neurol. 520, 2991–3012. 10.1002/cne.23103 [DOI] [PubMed] [Google Scholar]
- Petersen S. L., LaFlamme K. D. (1997). Progesterone increases levels of mu-opioid receptor mRNA in the preoptic area and arcuate nucleus of ovariectomized, estradiol-treated female rats. Brain Res. Mol. Brain Res. 52, 32–37. 10.1016/S0169-328X(97)00194-0 [DOI] [PubMed] [Google Scholar]
- Prasad P., Ogawa S., Parhar I. S. (2015). Role of serotonin in fish reproduction. Front. Neurosci. 9:195. 10.3389/fnins.2015.00195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthi K., Fropf R., Belfort G. M., Fitzmaurice H. L., McKinney R. M., Neve R. L., et al. (2011). Npas4 regulates a transcriptional program in CA3 required for contextual memory formation. Science 334, 1669–1675. 10.1126/science.1208049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanan N., Shen Y., Sarsfield S., Lemberger T., Schutz G., Linden D. J., et al. (2005). SRF mediates activity-induced gene expression and synaptic plasticity but not neuronal viability. Nat. Neurosci. 8, 759–767. 10.1038/nn1462 [DOI] [PubMed] [Google Scholar]
- Reiner A., Brauth S. E., Kitt C. A., Quirion R. (1989). Distribution of mu, delta, and kappa opiate receptor types in the forebrain and midbrain of pigeons. J. Comp. Neurol. 280, 359–382. 10.1002/cne.902800304 [DOI] [PubMed] [Google Scholar]
- Rink E., Wullimann M. F. (2001). The teleostean (zebrafish) dopaminergic system ascending to the subpallium (striatum) is located in the basal diencephalon (posterior tuberculum). Brain Res. 889, 316–330. 10.1016/S0006-8993(00)03174-7 [DOI] [PubMed] [Google Scholar]
- Sanchez-Simon F. M., Rodriguez R. E. (2008). Developmental expression and distribution of opioid receptors in zebrafish. Neuroscience 151, 129–137. 10.1016/j.neuroscience.2007.09.086 [DOI] [PubMed] [Google Scholar]
- Schroeder P. G., Sneddon L. U. (2017). Exploring the efficacy of immersion analgesics in zebrafish using an integrative approach. Appl. Anim. Behav. Sci. 187, 93–102. 10.1016/j.applanim.2016.12.003 [DOI] [Google Scholar]
- Šlamberová R., Rimanóczy Á., Cao D., Schindler C. J., Vathy I. (2005). Alterations of prenatal morphine exposure in μ-opioid receptor density in hypothalamic nuclei associated with sexual behavior. Brain Res. Bull. 65, 479–485. 10.1016/j.brainresbull.2005.02.030 [DOI] [PubMed] [Google Scholar]
- Spiegel I., Mardinly A. R., Gabel H. W., Bazinet J. E., Couch C. H., Tzeng C. P., et al. (2014). Npas4 regulates excitatory-inhibitory balance within neural circuits through cell-type-specific gene programs. Cell 157, 1216–1229. 10.1016/j.cell.2014.03.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C. W. (2009). The evolution of vertebrate opioid receptors. Front. Biosci. 14, 1247–1269. 10.2741/3306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart A., Wu N., Cachat J., et al. (2011). Pharmacological modulation of anxiety-like phenotypes in adult zebrafish behavioral models. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 1421–1431. 10.1016/j.pnpbp.2010.11.035 [DOI] [PubMed] [Google Scholar]
- Su Y.-L., Huang J., Wang N., Wang J.-Y., Luo F. (2012). The effects of morphine on basal neuronal activities in the lateral and medial pain pathways. Neurosci. Lett. 525, 173–178. 10.1016/j.neulet.2012.07.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Lin Y. (2016). Npas4: linking neuronal activity to memory. Trends Neurosci. 39, 264–275. 10.1016/j.tins.2016.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E., Pages C., Herve D., Girault J. A., Caboche J. (2004). Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur. J. Neurosci. 19, 1826–1836. 10.1111/j.1460-9568.2004.03278.x [DOI] [PubMed] [Google Scholar]
- Wullimann M. F., Rupp B., Reichert H. (1996). Neuroanatomy of the Zebrafish Brain: A Topologocal Atlas. Berlin: Birkhauser. [Google Scholar]
- Ye L., Allen W. E., Thompson K. R., et al. (2016). Wiring and molecular features of prefrontal ensembles representing distinct experiences. Cell 165, 1776–1788. 10.1016/j.cell.2016.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K. L., Rosenblum P. M., Peter R. E. (1991). In vitro release of gonadotropin-releasing hormone from the brain preoptic-anterior hypothalamic region and pituitary of female goldfish. Gen. Compar. Endocrinol. 81, 256–267. 10.1016/0016-6480(91)90010-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.