Summary
Polyethylene (PE) is one of the most widely used materials in the world, but it is virtually undegradable and quickly accumulates in nature, which may contaminate the environment. We utilized the cobalt-mediated radical copolymerization (CMRP) of ethylene and cyclic ketene acetals (CKAs) to effectively incorporate ester groups into PE backbone as cleavable structures to make PE-based copolymer degradable under mild conditions. The content of ethylene and ester units in the produced copolymer could be finely regulated by CKA concentration or ethylene pressure. Also, the copolymerization of ethylene and CKA with other functional vinyl monomers can produce functional and degradable PE-based copolymer. All the formed PE-based copolymers could degrade in the presence of trimethylamine (Et3N).
Subject Areas: Green Chemistry, Organic Chemistry, Polymers
Graphical Abstract
Highlights
-
•
We report cobalt-mediated copolymerization for inserting ester unit into PE backbone
-
•
The molecular weight of degraded product varied from several hundreds to thousands
-
•
This method provides access to a diverse range of degradable functional PE materials
Green Chemistry; Organic Chemistry; Polymers
Introduction
Polyethylene (PE) is vital to our society, affecting practically every aspect of modern life because it has so many advantages such as high strength, resistance to corrosion, low weight, and longevity (Eagan et al., 2017, Gao et al., 2019, Kermagoret et al., 2014, Klein and Briscoe, 1977). The longevity of plastics is one of their major advantages, but today it is also considered to be one of their great disadvantages (Author Anonymous, 2018, Devanand and Selser, 1990, Jambeck et al., 2015). Large amounts of these materials were used only once and then discarded, they hardly decomposed and could stay in environment for many years, which has caused a considerable part of wastes accumulating in nature and affecting both terrestrial and marine ecosystems (Author Anonymous, 2018, Lebreton et al., 2017). Now plastic wastes in natural environment have gained global attention (Cózar et al., 2017, Geyer et al., 2017, Haider et al., 2019b). Therefore, the synthesis of degradable PE-based copolymer that is kind to environment is a major goal of chemists. Ideally, introducing cleavable structures into the main chain of PE could make PE degradable (Tardy et al., 2017a, Tardy et al., 2017b). In 1980s, the copolymerizations of ethylene and carbon monoxide or vinyl ketone monomers have been employed to introduce carbonyl groups into the copolymer. The carbonyl group could undergo Norrish I and Norrish II photochemical reactions to cause chain scission (Ammala et al., 2011, Modi and Guillet, 1995, Scoponi et al., 1993). However, the incorporation of carbonyl group was very limited. Bailey et al. reported that the radical ring-opening polymerization of cyclic ketene acetals (CKAs) could yield polymers containing the same repeating unit as polylactones (Bailey and Gapud, 1984, Bailey et al., 1982); the ester units could degrade in the presence enzyme or base. The free radical copolymerization of ethylene and 2-methylene-1, 3-dioxepane (MDO) was also reported (Wu and Lenz, 1998); the copolymer with low molecular weight (Mn) was obtained, and the ester units distribution in the copolymer was essentially uniform and random. Recently, polyester formation from long-chain α, ω-diacids/diols, acyclic diene metathesis (ADMET) copolymerizations of dienes and nonfunctionalized α, ω-dienes with ester groups have also been reported. These routes provide access to PE-like degradable materials, but it is necessary for these methods to tailor the monomers (not directly from ethylene monomer) (Bauer et al., 2017, Haider et al., 2019a, Haider et al., 2019b, Steinbach and Wurm, 2015).
Cobalt-mediated radial polymerization (CMRP) based on the reversible deactivation of the growing radical chains by cobalt complexes is an efficient and versatile method to control the polymerization of vinyl monomers (Davis et al., 1995, Debuigne et al., 2005, Debuigne et al., 2009, Demarteau et al., 2019a, Guan, 2002, Hsu et al., 2014, Li et al., 2008, Miao et al., 2014, Peng et al., 2009, Wang et al., 2017). Reversible termination (RT) and degenerate transfer (DT) are two mechanisms that occur in CMRP. RT involves the trap and release of the radical chain via reversible metal-carbon bond homolysis and occurs when the total radical concentration is less than that of the trapping cobalt species, whereas DT occurs under higher radical concentration in which a radical species displaces the trapped radical bound to the metal (Debuigne et al., 2009, Li et al., 2008, Wayland et al., 2006). Cobalt complexes could be used to control the polymerization of both low-activated monomers such as vinyl acetate (VAc) and N-vinylpyrrolidone (NVP) (Debuigne et al., 2005, Debuigne et al., 2011, Demarteau et al., 2017, Hsu et al., 2014, Kermagoret et al., 2014, Wang et al., 2017) and more-activated monomers such as methyl acrylate (MA) with high living character (Liao et al., 2013, Lu et al., 2004). Up to now, only Co(acac)2 has been reported to be able to control the copolymerization of ethylene and vinyl monomers (Bryaskova et al., 2007, Demarteau et al., 2018, Demarteau et al., 2019b, Kermagoret et al., 2014). Herein, we reported the preparation of degradable PE-based copolymers with well-tuned ester incorporation by CoⅡ(Salen*)-mediated radical copolymerization of ethylene and CKAs under mild conditions; the ring-opening reaction of CKAs could effectively incorporate ester structures into copolymer backbone to ensure degradation.
Results and Discussion
Homo-Polymerization of MDO and Homo-Polymerization of Ethylene
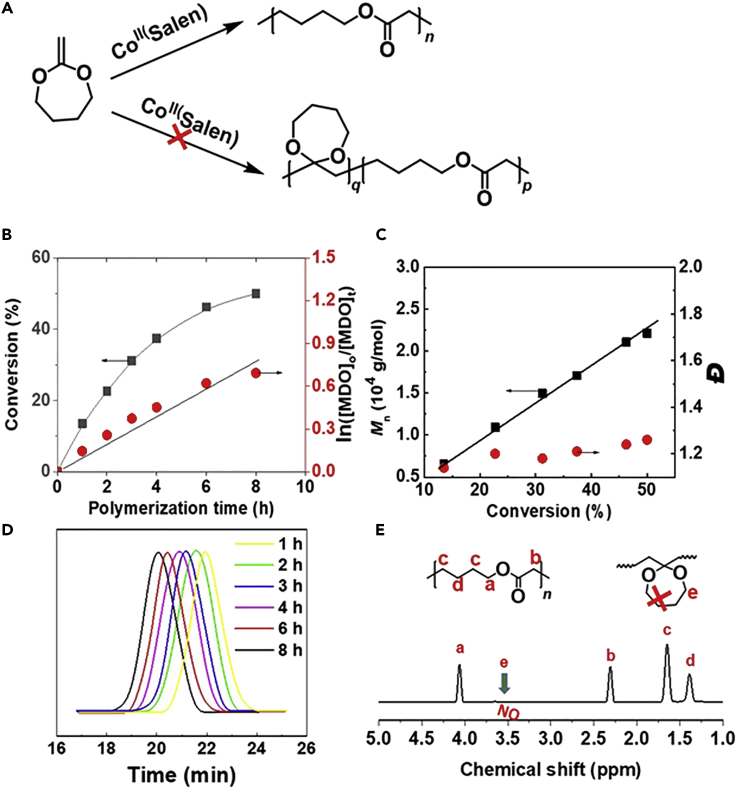
The MDO monomer was commonly used to be copolymerized with vinyl monomers via radical mechanism for preparing degradable polymers (Hedir et al., 2014, Hedir et al., 2015, Hedir et al., 2017, Undin et al., 2014); the product of ring-opening polymerization of MDO has ester group in its backbone, which could easily degrade in acidic or basic conditions (Agarwal, 2010, Hill et al., 2017, Hill et al., 2018, Tardy et al., 2017b, Undin et al., 2012). The MDO propagating radicals lack stabilized group and generally result in unfavorable reactivity ratios toward more-activated monomers, usually leading to very limited incorporation in the final copolymer when copolymerizing with more-activated monomers (Seema and Liqun, 2009, Undin et al., 2013); however, the copolymerization of MDO and less-activated monomers (e.g., vinyl acetate, vinyl ethers) could yield statistical copolymers (Hedir et al., 2017, Tardy et al., 2017a). In our work, CoⅡ(Salen*)-mediated radical homopolymerization of MDO using AIBN as the initiator at 75°C was carried out. Generally, MDO is the isomer of the corresponding lactones, which can undergo radical addition at the double bond with subsequent possibilities of either ring opening or ring retaining or a combination of the two (Agarwal, 2007, Bailey et al., 1982, Carter et al., 2016), and the corresponding signals of acetal CH2 of MDO (ring-retaining part) in polymer is at 3.5 ppm in the 1H NMR spectrum. But, there was no signal at 3.5 ppm in 1H NMR of the resulting polymer produced via CMRP of MDO (as shown in Figure 1E), which indicates that CMRP of MDO gave only ring-opening product without any ring-retaining product as shown in Figures 1A and 1E (Carter et al., 2016). The kinetics of the homopolymerization of MDO mediated by CoⅡ(Salen*) was also investigated, which exhibited living polymerization characters (Figures 1B–1D); the dispersity (Đ) of the resulting PMDO is low, and the molecular weight (Mn) of the resulting polymer increased almost linearly with MDO conversion (Figure 2C). The homopolymerization of ethylene mediated by CoII(Salen*) was also carried out with AIBN as the initiator and dimethyl carbonate (DMC) as the solvent under the conditions of DMC (1.8 mL), CoII(Salen*) (6 mg, 0.01 mmol), and AIBN (10.7 mg, 0.065 mmol) at 75°C (Grau et al., 2011), and PE was successfully obtained and the results are shown in Figure S4 and Table S1. Mn of the obtained PE increased almost linearly with the yield, indicating that the polymerization of ethylene is controlled (Wolpers et al., 2019).
Figure 1.
Homo-Polymerization of MDO Mediated by CoII(Salen*)
Conditions: MDO (5.7 g, 50.0 mmol), CoII (Salen*) (60.4 mg, 0.1 mmol), AIBN (82.0 mg, 0.5 mmol), 75°C.
(A) The scheme of ring-opening radical homo-polymerization of MDO mediated by CoII(Salen*).
(B) The evolution of monomer conversion and ln([MDO]0/[MDO]t) with polymerization time.
(C) The variation of Mn and Đ for PMDO with MDO conversion.
(D) GPC (THF, 35°C) traces of PMDO at different polymerization times.
(E) 1H NMR spectrum of the produced PMDO.
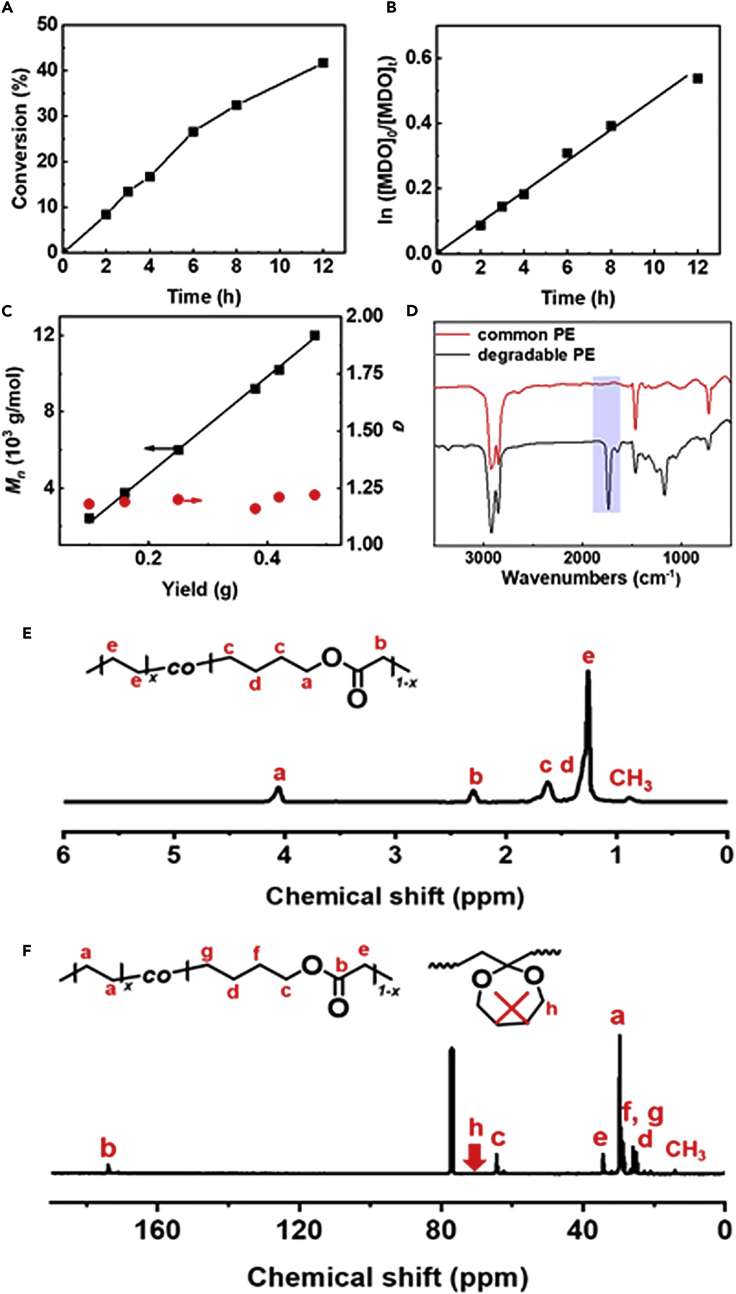
Figure 2.
The Copolymerization of Ethylene and MDO Mediated by CoⅡ(Salen*)
Conditions: MDO (0.6 mL), DMC (1.8 mL), ethylene (30 bar), CoⅡ(Salen*) (6.0 mg, 0.01 mmol), AIBN (10.7 mg, 0.065 mmol), 75°C.
(A) The evolution of MDO conversion with polymerization time.
(B) The evolution of ln([MDO]0/[MDO]t) with polymerization time.
(C) The variation of Mn and Đ for produced PE-based copolymer with ester structures in the backbone with copolymer yield.
(D) FTIR spectra of common PE and produced PE-based copolymer with ester structures in the backbone.
(E) 1H NMR spectrum of produced PE-based copolymer with 76.9% ethylene content.
(F) 13C NMR spectrum of produced PE-based copolymer with 76.9% ethylene content.
Copolymerization of Ethylene and MDO Using CMRP Method
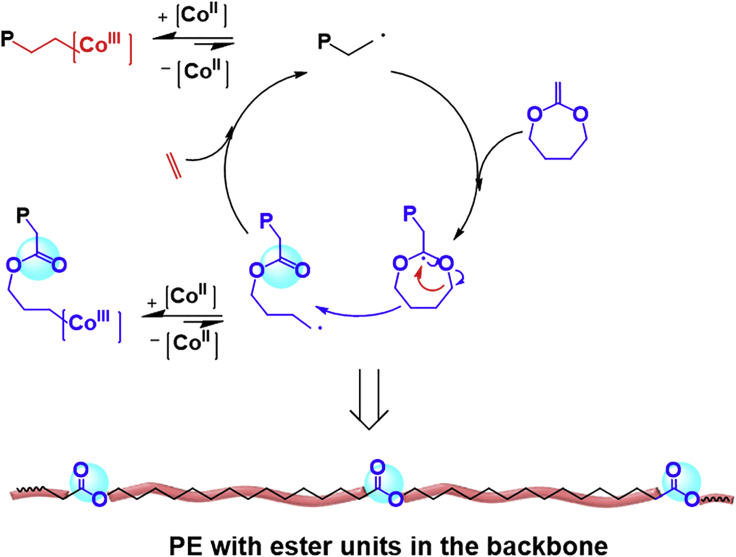
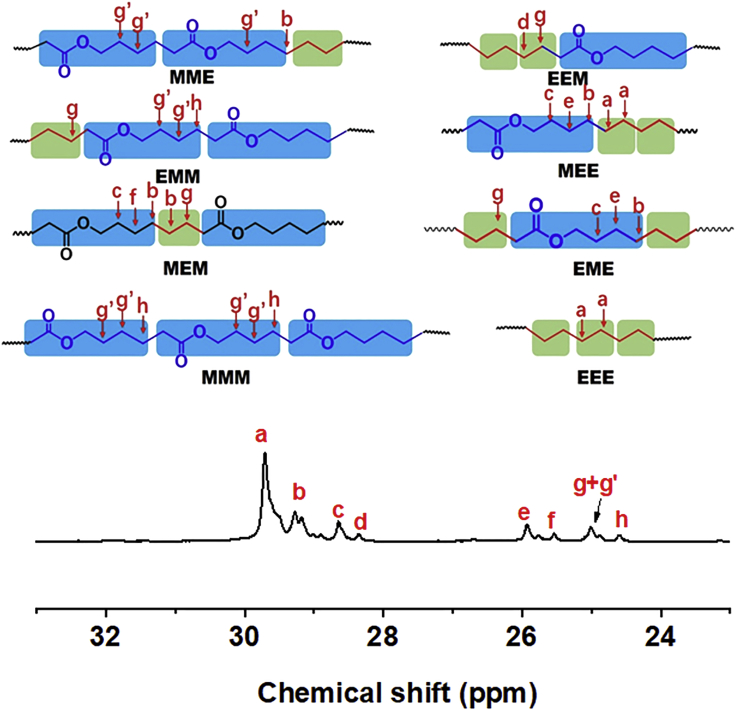
The concentration of radical initiator is critical to the CMRP of less-activated monomers since it dominates the radical concentration in the polymerization system. A higher radical concentration would give a faster and more economical polymerization process, but a lower radical concentration provides a better control to the polymeric product. According to the previously published research, the CMRP of vinyl acetate could be well controlled under the initiator/Co complex molar ratio range of 1–10 (Liao et al., 2013, Lu et al., 2004, Zhao et al., 2015a, Zhao et al., 2015b). Herein, the CMRP of ethylene and MDO was performed at a molar ratio of CoII(Salen*):AIBN = 1:6.5 according to previous reports on CMRP (Liao et al., 2013). MDO was successfully copolymerized with VAc mediated by Co(acac)2, and a statistical copolymer was obtained (Ding et al., 2016). In the CMRP of ethylene in the presence of MDO, the growing radical can attack the double bond of MDO and immediately the MDO ring opens to form an ester unit in the main chain and another growing chain radical, which could further react with either ethylene or MDO (Scheme 1). Therefore, the CMRP of ethylene and MDO could provide a valuable route to introduce degradable ester units into the PE backbone under the mild conditions. After 22 h of copolymerization of MDO and ethylene mediated by CoII(Salen*) in DMC at 75°C, the color turned from red of the initial mixture to dark green, which indicated that CoⅢ complex formed from CoⅡ complex after trapping the propagating radicals (Debuigne et al., 2009, Kermagoret et al., 2014). The CMRP of ethylene and MDO was carried out at 30 bar of ethylene pressure and 1:3 volume ratio of MDO: DMC (Figures 2A–2C). The first order kinetic plot (Figure 2B) could be observed. The Mn of the obtained polymers increased almost linearly with the copolymer yield (Figure 2C), and Đ was low throughout the copolymerization (Figure 2C). Fourier transform infrared spectroscopy (FTIR) spectra of PE and PE-based copolymer with ester structures in the backbone are shown in Figure 2D; the absorptions at 2,850–2,950 cm−1 were assigned to the stretching mode of –CH2–CH2– in the PE backbone. The absorptions at 1,480 cm−1 came from the methylene scissors motion. Compared with the FTIR spectrum of PE, the FT-IR spectrum of PE-based copolymer with ester structures in the backbone showed a strong absorption at 1,738 cm−1, which is attributed to the stretching vibration of C=O of the ester groups originating from the ring-opening structure of MDO, and the absorption at 1,166 cm−1 came from the C–O structure. We further used 1H NMR to characterize the structure of the PE-based copolymer with 76.9% ethylene content, and the results are shown in Figure 2E. The signal at 4.06 ppm came from the methylene protons of –COOCH2 structure, and the signal at 2.29 ppm was assigned to the methylene protons of –CH2COO structure originating from MDO ring-opening structure. The signals for all other protons in the copolymer were present between 1.77 and 0.80 ppm. The strong signal at 1.25 ppm was typical –CH2–CH2 protons in the backbone. The amount of ethylene structures in the copolymer could be (It-6)/4 while setting the integral value of the peak around 4.06 ppm as 2 and the integral values from 1.77 to 0.80 ppm as It; the content of ethylene could be further determined by the formula [(It −6)/4]/[1+(It −6)/4]. The signal around 0.88 ppm was assigned to –CH3 groups of the branched ethylene units; the medium signals indicated that the produced copolymers have branched chains, and the degree of branching (DB, CH3 branches/1000 C atoms) is listed in Table 1. The branched structure could also be confirmed in the 13C NMR spectrum (Figure 2F); a medium signal at 14.1 ppm is attributed to the –CH3 groups in the branched chain. The DB of the copolymer obtained by the CMRP method was close to the PE obtained by conventional free radical mechanism (determined to be 45) but was a litter higher than the EVA copolymers obtained using Co(acac)2, which may result from that the polymerization temperature of CMRP is higher than that of the previously reported work (Kermagoret et al., 2014). Generally, higher polymerization temperature would produce PE with higher DB. The existence of ester units could also be confirmed by 13C NMR spectrum (Figure 2F). The signal at 174.0 ppm was assigned to the carbon in –COO groups originating from the MDO ring-opening structure; the absence of a signal around 70.4 ppm, corresponding to a ketal carbon, indicated that MDO underwent ring-opening reaction and no ring remaining products formed. Furthermore, the 1H COSY NMR spectrum could also prove the existence of ester structure in the PE backbone. The 1H DOSY NMR spectrum of the produced polymer gave only single diffusion coefficiency (Figure S5), indicating that the produced polymer has one kind of polymer chain, is the copolymer, but not the mixture of PE and PMDO. On the other hand, we used 13C NMR spectrum and HSQC NMR spectrum to identify the major sequence structural units in the produced PE-based copolymers (Figures 3 and S8). The strong signal at 29.7 ppm is attributed to methylene carbon in linear PE corresponding to EEE units in this case (Savant et al., 2007). Signals at 29.1 and 29.3 ppm are coupled with the 1H NMR resonance at 1.64 ppm in the HSQC spectrum (Figure S8) and are assigned to the carbon in –COOCH2CH2CH2CH2–CH2CH2 (ME) structure coming from MEE or EME; it is clear that the signal corresponding to EEE and ME structure are strong, whereas the signal corresponding to MMM and MEM units are weak (Figure 3). The presence of these signals could confirm the random incorporation of the MDO ring-opening structure in the copolymer backbone. The living character of the CMRP process was further confirmed by the chain extension experiment as shown in Scheme S1. CoIII(Salen*)-P(MDO-co-E) with Mn of 11,000 g/mol and Đ of 1.15 was obtained after 22 h of polymerization under 10 bar of ethylene pressure, 6 mg of CoII(Salen*), 1 mL of total mixture, and molar ratio of CoII(Salen*):AIBN:MDO = 1:6.5:526 at 75°C. The obtained CoIII(Salen*)-P(MDO-co-E) was further used as macro-initiator for the bulk polymerization of VAc at 75°C. After 20 h of polymerization, the block copolymer CoIII(Salen*)-PVAc-b-P(MDO-co-E) was obtained with Mn of 35,300 g/mol and Đ of 1.26, as shown in Figures S6 and S7, which indicates that the CMRP of ethylene and MDO is living radical polymerization.
Scheme 1.
Formation of PE-Based Copolymer with Ester Structures in the Backbone via the CMRP of Ethylene and MDO
Table 1.
Copolymerization of Ethylene and MDO under Different Volume Ratios of MDO:DMC and Ethylene Pressure
| Entry | P (bar) | MDO:DMC (v:v) | Xethylenea (%) | Mnb (103 G/mol) | Đ | Yield (g) | DBc |
|---|---|---|---|---|---|---|---|
| 1 | 10 | 1:3 | 64.4 | 10.1 | 1.17 | 0.43 | 42 |
| 2 | 20 | 1:3 | 68.3 | 11.2 | 1.19 | 0.45 | 41 |
| 3 | 30 | 1:3 | 76.9 | 12.5 | 1.21 | 0.54 | 43 |
| 4 | 40 | 1:1 | 77.0 | 14.8 | 1.22 | 0.83 | 42 |
| 5 | 40 | 1:2 | 88.8 | 13.4 | 1.20 | 0.81 | 44 |
| 6 | 40 | 1:3 | 89.8 | 9.5 | 1.16 | 0.74 | 40 |
| 7 | 40 | 1:4 | 92.6 | 7.6 | 1.21 | 0.67 | 45 |
Conditions: MDO (0.6 mL), CoⅡ(Salen*) (6.0 mg, 0.01 mmol), AIBN (10.7 mg, 0.065 mmol), 75°C, 22 h.
Ethylene content was calculated based on 1H NMR spectra.
Mn was determined by GPC (TCB, 150°C).
Degree of branching, calculated based on 1H NMR spectra.
Figure 3.
13C NMR Spectrum of the Major Sequence Structure Units in Produced PE-Based Copolymer with 76.9% Ethylene Content
Regulation of Ethylene Content in the Copolymer
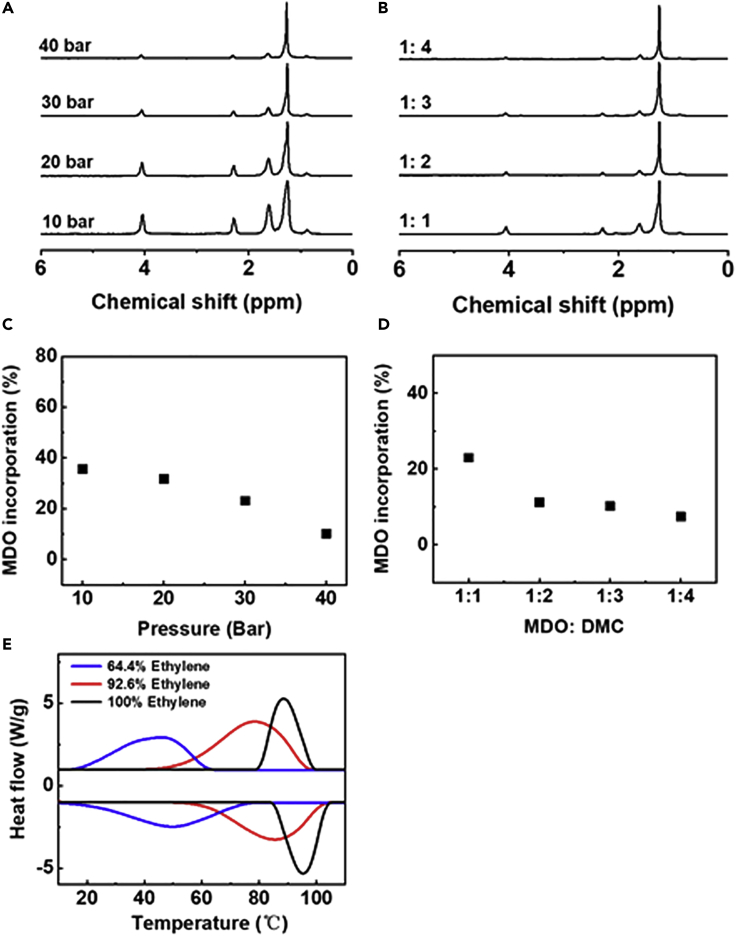
The pressure of ethylene and the concentration of MDO have an effect on the ethylene content in the produced copolymer. It is clear that by increasing ethylene pressure from 10 to 40 bar, the ethylene content in the produced copolymers increased from 64.4% to 89.8%, as shown in Table 1, and the intensity of the signal at 4.06 ppm decreased obviously when increasing ethylene pressure (Figures 4A and 4C). At higher ethylene pressure, more ethylene could dissolve in the mixture and more ethylene participated in the copolymerization; the ethylene content in the copolymers increased consequently. The ethylene content in the copolymer also depends on MDO concentration. At 40 bar of ethylene pressure with 0.6 mL of DMC, the ethylene content was around 77.0%, but it could reach up to 92.6% when quadrupling the solvent, the intensity of the signal at 4.06 ppm also decreased (Figures 4B and 4D). Ethylene concentration remained unchanged under the specific pressure and temperature; the concentration of MDO in DMC decreased when quadrupling the solvent. The lower concentration of MDO in DMC indicated the higher molar ratio of ethylene to MDO in the initial polymerization mixture, and ethylene content in copolymer is high consequently. The Mn of the copolymers and ethylene content in the produced copolymers are shown in Table 1; the Mn could reach up to 10,000 g/mol with low Đ. Because the CMRP of ethylene and MDO is living polymerization, the Mn of the produced PE-based copolymers could be further increased by decreasing the amount of CoⅡ(Salen*). When the amounts of CoⅡ(Salen*) and AIBN were halved and other conditions remained unchanged as Entry 5, Table 1, the Mn of the formed PE-based copolymer was nearly doubled to 27,700 g/mol, whereas the content of ethylene and yield of the copolymer did not vary too much, as shown in Figure S9 and Table S2. The polymerization kinetics changed very little in the presence of MDO; Mn and DB of the PE-based copolymer are similar to those of PE produced via the CMRP method (Figures 2C and S4 and Tables 1 and S1). The Mn could reach up to 10,000 g/mol and increased with polymer yield; the DB was around 40–50. However, the solubility of PE-based copolymer with ester structures in DMC is much better than that of PE. The incorporation of ester structures may destroy the crystallization of PE and caused the melting temperature (Tm) of the produced PE-based copolymer to decrease. Tm of common PE and the PE-based copolymers are shown in Figure 4E; Tm decreased with the increase of ester structures in the PE-based copolymer chain. The PE-based copolymer with 92.6% ethylene content has the Tm of 86.2°C, which is close to the Tm (92.4°C) of PE, but the melting range is broader than PE.
Figure 4.
The Effect on Ethylene Content in the Produced Polymer
Conditions: MDO (0.6 mL), CoⅡ(Salen*) (6.0 mg, 0.01 mmol), AIBN (10.7 mg, 0.065 mmol), 75°C, 22 h.
(A) 1H NMR spectra of produced PE-based copolymers prepared under different ethylene pressure (10–40 bar), MDO:DMC = 1:3.
(B) 1H NMR spectra of produced PE-based copolymers prepared under different volume ratios of MDO:DMC (1:1 to 1:4), ethylene pressure was 40 bar.
(C) MDO incorporation in the produced copolymer under different ethylene pressure.
(D) MDO incorporation in the produced copolymer under different volume ratios of MDO: DMC.
(E) DSC thermograms for PE-based copolymer with ester structures and common PE.
Copolymerization of Ethylene and Other CKA
We also prepared other CKA, such as 2-methylene-1, 3, 6-trioxocane (MTC), to copolymerize with ethylene. The homopolymerization of MTC using this CMRP method was conducted. The obtained PMTC was analyzed using 1H NMR as shown in Figure S10. The signal at 4.27 ppm was assigned to the –COOCH2, the signals between 3.71 and 3.46 ppm were assigned to the CH2OCH2, and the signal at 2.43 ppm was assigned to the –CH2COO; the existence of these signals proved that MTC underwent ring-opening reaction during the polymerization. Similar to the mechanism of the CMRP of ethylene and MDO, in the copolymerization of MTC and ethylene, MTC could also ring open to introduce ester units as well as ether units into the polymer backbone without any ring remaining reactions when the double bond of MTC was attacked by a radical. The MTC ring-opening structure obviously exists in the 1H NMR spectrum of the resulting P(MTC-co-E); the strong signal around 1.25 ppm came from –CH2–CH2– structure of PE (Figure S11), and the ethylene content was determined to be 88.4% under the conditions of MTC (0.6 mL), DMC (1.8 mL), CoII(Salen*) (6.0 mg, 0.01 mmol), 30 bar of ethylene pressure, and 75°C for 22 h. The CMRP of MTC and ethylene was also carried out at 75°C with AIBN as the initiator at 30 bar of ethylene pressure, MTC (0.6 mL) and DMC (1.8 mL); the results are shown in Figure S12. The Mn of the obtained copolymer increased almost linearly with the copolymer yield, and Đ was low throughout the copolymerization (Figure S12C); therefore, CoⅡ(Salen*) could also mediate the copolymerization of MTC and ethylene.
Functionalization of Degradable PE-Based Copolymer
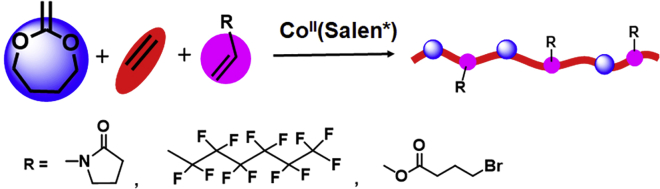
The copolymerization of MDO and ethylene with other functional vinyl monomers could offer a promising method to design functional PEs with ester structures in the backbone (Scheme 2). Fluoropolymers have particular properties, such as chemical inertness, high thermal stability, and hydrophobicity. Introducing fluoro-containing units into the PE chain could significantly change its properties. The copolymerization of MDO, ethylene, and perfluorohexylethylene (PFHE) was carried out as shown in Scheme 2 and Figure S13. Gel permeation chromatography (GPC) (THF, 35°C) results showed unimodal peak for all samples, and the Mn of the formed fluoro-containing PE could reach up to 10,000 g/mol with low Đ. We also changed the concentration of PFHE and ethylene pressure to regulate the content of each component in the copolymer, and the results are listed in Table 2. In contrast to the hydrophobicity of poly(perfluorohexylethylene), poly(N-vinyl -pyrrolidone) exhibits hydrophilicity. The copolymerization of ethylene, MDO, and N-vinyl pyrrolidone (NVP) was also conducted. After 22 h of polymerization, the mixture became dark green and was of high viscosity; the pyrrolidone-containing PE copolymers were analyzed using 1H NMR and GPC (THF, 35°C). Ethylene pressure was also changed to regulate the composition of the produced copolymers, and the results are shown in Table 3 and Figure S14. The content of each component in the copolymer could be regulated by ethylene pressure or monomer concentration; higher pressure would produce copolymer with high ethylene content. The surface properties of the produced copolymers could be significantly varied after introducing different hydrophilic groups or hydrophobic groups into PE-based copolymers. For common PE, the contact angle with water was 115.5°, whereas the PE-based copolymer with 7.4% MDO content in the backbone has a contact angle of 107.9°. The contact angle could be raised to 126.7° for the PE copolymer with 17.8% PFHE and 20.1% MDO contents, whereas it could decrease to 96.9° for the copolymer with 15.6% NVP and 8.0% MDO contents (Figure S15). Also, the copolymerization of ethylene, MDO, and vinyl bromobutanoate (VBr) could yield the copolymer (P(MDO-co-E-co-VBr)) with pendent bromine functional groups that are able to be modified via post-polymerization modification as shown in Scheme S2 and Figures S16 and S17 under the conditions of MDO (0.6 mL), VBr (0.3 mL), DMC (1.2 mL), CoII(Salen*) (6 mg, 0.01 mmol), AIBN (10.7 mg, 0.065 mmol), 30 bar of ethylene pressure, and 75°C for 22 h; the contents were determined to be 15.1% for MDO, 43.3% for VBr, and 41.6% for ethylene, respectively. The side bromine unit of the resulting P(MDO-co-E-co-VBr) can be further replaced by azide group after reacting with NaN3 in DMF for 48 h to yield P(MDO-co-E-co-VN3). In the 1H NMR spectrum (Figure S16), a clear shift of CH2–Br (δ = 3.50 ppm) to the CH2–N3 (δ = 3.40 ppm) was observed, indicating that all bromine units have been completely replaced by azide group. GPC (THF, 35°C) results showed almost the same curves for copolymer before and after azidation (Figure S17). Via “click” chemistry of 1, 3-dipolar cycloaddition reaction of azides with alkynes, glycol units can be easily linked onto the PE copolymer chain. Propynyl glycol acrylate and the copolymer P(MDO-co-E-co-VN3) were dissolved in DMF and allowed for reacting at 80°C; the appearance of new resonances at 8.25 (the triazole proton) and 3.66 ppm coming from propynyl glycol acrylate units and the absence of the resonance at δ = 3.40 ppm (CH2–N3), which shifted to 4.50 ppm after connection with the triazole ring, indicated successful linking of the propynyl glycol acrylate group onto the PE copolymer. GPC (THF, 35°C) results showed that the resulting copolymer after “click” has an increase in Mn (Figure S17). The advantage of this method is the versatility in the approaches that can be used to prepare the functional copolymers.
Scheme 2.
The CMRP of Ethylene, MDO, and Functional Vinyl Monomers
Table 2.
Copolymerization of Ethylene, MDO, and PFHE
| Entry | PFHE (mL) | Pressure (bar) | Contenta (%) |
Mnb (103 G/mol) | Đ | Yield (g) | ||
|---|---|---|---|---|---|---|---|---|
| E | PFHE | MDO | ||||||
| 1 | 0.3 | 20 | 47.6 | 15.3 | 37.1 | 10.6 | 1.17 | 1.05 |
| 2 | 0.6 | 20 | 62.1 | 17.8 | 20.1 | 14.1 | 1.21 | 1.31 |
| 3 | 0.6 | 40 | 69.8 | 13.6 | 16.6 | 17.8 | 1.18 | 1.78 |
Conditions: MDO (0.6mL), DMC (1.2 mL), CoⅡ(Salen*) (6.0 mg, 0.01 mmol), AIBN (10.7 mg, 0.065 mmol), 75°C, 22 h.
Monomer content, calculated based on 1H NMR spectra.
Determined by GPC (THF, 35°C).
Table 3.
Copolymerization of Ethylene, MDO, and NVP
| Entry | Pressure (bar) | Contenta (%) |
Mnb (103 G/mol) | Đ | Yield (g) | ||
|---|---|---|---|---|---|---|---|
| E | NVP | MDO | |||||
| 1 | 40 | 76.4 | 15.6 | 8.0 | 8.6 | 1.21 | 0.78 |
| 2 | 20 | 53.9 | 30.1 | 16.0 | 6.6 | 1.18 | 0.52 |
Conditions: MDO (0.6mL), NVP (0.6 mL), DMC (1.2 mL), CoⅡ(Salen*) (6.0 mg, 0.01 mmol), AIBN (10.7 mg, 0.065 mmol), 75°C, 22 h.
Monomer content, calculated based on 1H NMR spectra.
Determined by GPC (THF, 35°C).
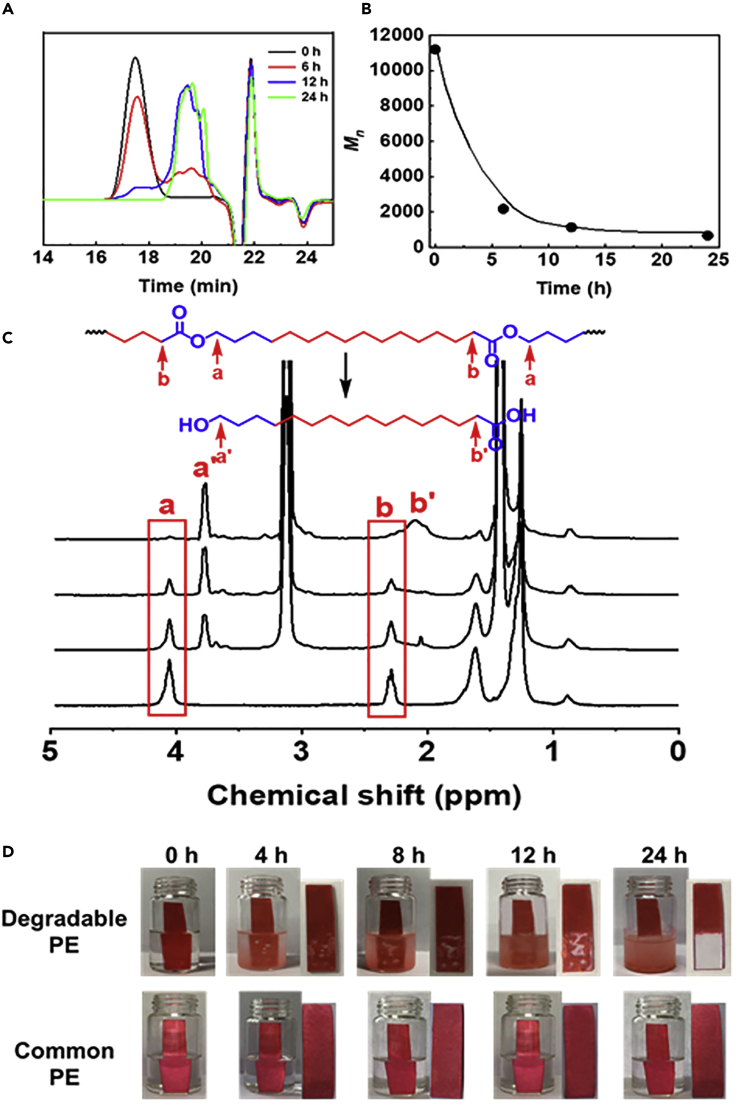
Degradation of the PE-Based Copolymer with 68.3% Ethylene Content
The degradation behavior was investigated by the hydrolysis of PE-based copolymer in mixed triethylamine (Et3N) and chloroform (Mattsson et al., 2007). When 40 mg of PE-based copolymer with 68.3% ethylene content (Entry 2, Table 1) was dissolved in the solution of 1 mL Et3N in 4 mL of chloroform by heating to 70°C in the presence of small amount of water, the intensity of the resonance at 4.06 ppm (–CH2OOC–, a) decreased obviously and a new resonance at 3.75 ppm (–CH2OH, a᾽) was present, and the intensity of the resonance at 2.3 ppm (–CH2COOCH2–, b) decreased obviously and a new resonance at 2.1 ppm (–CH2COOH, b᾽) was present, which was attributed to the degradation of ester group into hydroxyl group, since the resonance of CH2 group connected to hydroxyl group usually occurred at 3.75 ppm. When prolonging the reaction time, the resonance at 4.06 ppm became weaker and the resonance at 3.75 ppm became stronger obviously as shown in Figure 5C. After 24 h, the resonance at 4.06 ppm was almost absent (Figure 5C), which could prove that the ester group was fully degraded. GPC (TCB, 150°C) results showed that PE with Mn of 11,200 g/mol has degraded into the products with Mn of 670 g/mol in 24 h (Figures 5A, 5B, and S18). We also prepared films of common PE and degradable PE films with purple color; subsequently, two films emerged in the mixed solvent of CH3OH and Et3N in presence of small amount of water. It is very clear that the PE film completely degraded and the solvent became purple from colorless, whereas the common PE film and the solvent remained unchanged as shown in Figure 5D.
Figure 5.
The Degradation of PE-Based Copolymer with Ester Structures in the Backbone
(A) GPC (TCB, 150°C) curves of PE-based copolymer with ester structures in the backbone treated with Et3N at different times.
(B) Percent decrease of Mn of PE-based copolymer with ester structures in the backbone treated with Et3N at different times.
(C) 1H NMR spectra of the copolymer treated with Et3N at different times.
(D) The picture of film of PE-based copolymer with ester structures in the backbone and common PE film treated with Et3N at different times.
Conclusion
We utilized the CMRP method to copolymerize ethylene and CKAs to effectively incorporate ester groups into PE backbone for making PE-based copolymer that degrades under mild conditions. Ethylene content in the copolymer was regulated by ethylene pressure or MDO concentration, and it could be easily tuned in a wide range. Also, the functionalization of the PE-based copolymer was investigated; the copolymerization of ethylene and MDO with other functional vinyl monomers could produce functional and degradable PE-based copolymer. All the formed PE-based copolymer could degrade. This CMRP method would provide access to a diverse range of degradable and functional polyolefin materials.
Limitations of the Study
Although the degradable PE-based copolymer with ester structures in the backbone was successfully obtained with the ethylene content varying in a wide range, it is still a challenge to further improve the Mn of the copolymer for affording PE-based copolymers with good mechanical properties. Also, the degradation under natural environment need to be investigated in the future.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We acknowledge funding support from the National Key R&D Program of China (2017YFA0205600) and the National Natural Science Foundation of China (Grant No. 51625305, 21525420, 51873202, and 21774113).
Author Contributions
T.Z. and W.Y. conducted the experiments. C.H., C.C., and Y.Y. designed the experiments, prepared the manuscript, and wrote the paper. G.C, X.N., Z.Z., and L.X. have done some characterization on the polymers.
Declaration of Interests
The authors declare no competing interests.
Published: March 27, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.100904.
Contributor Information
Ze Zhang, Email: zze320@mail.ustc.edu.cn.
Chunyan Hong, Email: hongcy@ustc.edu.cn.
Changle Chen, Email: changle@ustc.edu.cn.
Yezi You, Email: yzyou@ustc.edu.cn.
Supplemental Information
References
- Author Anonymous The future of plastic. Nat. Commun. 2018;9:2157. doi: 10.1038/s41467-018-04565-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S. Microstructural characterisation and properties evaluation of poly (methyl methacrylate-co-ester) s. Polym. J. 2007;39:163. [Google Scholar]
- Agarwal S. Chemistry, chances and limitations of the radical ring-opening polymerization of cyclic ketene acetals for the synthesis of degradable polyesters. Polym. Chem. 2010;1:953–964. [Google Scholar]
- Ammala A., Bateman S., Dean K., Petinakis E., Sangwan P., Wong S., Yuan Q., Yu L., Patrick C., Leong K.H. An overview of degradable and biodegradable polyolefins. Prog. Polym. Sci. 2011;36:1015–1049. [Google Scholar]
- Bailey W.J., Gapud B. Synthesis of biodegradable polyethylene. Polym. Prepr. 1984;25:58–59. [Google Scholar]
- Bailey W.J., Ni Z., Wu S.R. Synthesis of poly-ε-caprolactone via a free radical mechanism. Free radical ring-opening polymerization of 2-methylene-1, 3-dioxepane. J. Polym. Sci. Polym. Chem. Ed. 1982;20:3021–3030. [Google Scholar]
- Bauer K.N., Tee H.T., Velencoso M.M., Wurm F.R. Main-chain poly(phosphoester)s: history, syntheses, degradation, bio-and flame-retardant applications. Prog. Polym. Sci. 2017;73:61–122. [Google Scholar]
- Bryaskova R., Willet N., Degée P., Dubois P., Jérôme R., Detrembleur C. Copolymerization of vinyl acetate with 1-octene and ethylene by cobalt-mediated radical polymerization. J. Polym. Sci. A Polym. Chem. 2007;45:2532–2542. [Google Scholar]
- Carter M.C.D., Jennings J., Appadoo V., Lynn D.M. Synthesis and characterization of backbone degradable azlactone-functionalized polymers. Macromolecules. 2016;49:5514–5526. [Google Scholar]
- Cózar A., Martí E., Duarte C.M., García-de-Lomas J., Van Sebille E., Ballatore T.J., Eguíluz V.M., González-Gordillo J.I., Pedrotti M.L., Echevarría F. The arctic ocean as a dead end for floating plastics in the North Atlantic branch of the thermohaline circulation. Sci. Adv. 2017;3:e1600582. doi: 10.1126/sciadv.1600582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis T.P., Kukulj D., Haddleton D.M., Maloney D.R. Cobalt-mediated free-radical polymerization of acrylic-monomers. Trends Polym. Sci. 1995;3:365–373. [Google Scholar]
- Debuigne A., Caille J.R., Jérôme R. Highly efficient cobalt-mediated radical polymerization of vinyl acetate. Angew. Chem. Int. Ed. 2005;44:1101–1104. doi: 10.1002/anie.200461333. [DOI] [PubMed] [Google Scholar]
- Debuigne A., Poli R., Jérôme C., Jérôme R., Detrembleur C. Overview of cobalt-mediated radical polymerization: roots, state of the art and future prospects. Prog. Polym. Sci. 2009;34:211–239. [Google Scholar]
- Debuigne A., Schoumacher M., Willet N., Riva R., Zhu X., Rutten S., Jerome C., Detrembleur C. New functional poly(N-vinylpyrrolidone) based (co)polymers via photoinitiated cobalt-mediated radical polymerization. Chem. Commun. (Camb.) 2011;47:12703–12705. doi: 10.1039/c1cc15471k. [DOI] [PubMed] [Google Scholar]
- Demarteau J., Améduri B., Ladmiral V., Mees M.A., Hoogenboom R., Debuigne A., Detrembleur C. Controlled synthesis of fluorinated copolymers via cobalt-mediated radical copolymerization of perfluorohexylethylene and vinyl acetate. Macromolecules. 2017;50:3750–3760. [Google Scholar]
- Demarteau J., De Winter J., Detrembleur C., Debuigne A. Ethylene/vinyl acetate-based macrocycles via organometallic-mediated radical polymerization and CuAAC ‘click’ reaction. Polym. Chem. 2018;9:273–278. [Google Scholar]
- Demarteau J., Debuigne A., Detrembleur C. Organocobalt complexes as sources of carbon-centered radicals for organic and polymer chemistries. Chem. Rev. 2019;119:6906–6955. doi: 10.1021/acs.chemrev.8b00715. [DOI] [PubMed] [Google Scholar]
- Demarteau J., Scholten P.B.V., Kermagoret A., De Winter J., Meier M.A.R., Monteil V., Debuigne A., Detrembleur C. Functional polyethylene (PE) and PE-based block copolymers by organometallic-mediated radical polymerization. Macromolecules. 2019;52:9053–9063. [Google Scholar]
- Devanand K., Selser J.C. Polyethylene oxide does not necessarily aggregate in water. Nature. 1990;343:739–741. [Google Scholar]
- Ding D., Pan X., Zhang Z., Li N., Zhu J., Zhu X. A degradable copolymer of 2-methylene-1, 3-dioxepane and vinyl acetate by photo-induced cobalt-mediated radical polymerization. Polym. Chem. 2016;7:5258–5264. [Google Scholar]
- Eagan J.M., Xu J., Di Girolamo R., Thurber C.M., Macosko C.W., LaPointe A.M., Bates F.S., Coates G.W. Combining polyethylene and polypropylene: enhanced performance with PE/iPP multiblock polymers. Science. 2017;355:814–816. doi: 10.1126/science.aah5744. [DOI] [PubMed] [Google Scholar]
- Gao Y.S., Chen J.Z., Wang Y., Pickens D.B., Motta A., Wang Q.J., Chung Y.W., Lohr T.L., Marks T.J. Highly branched polyethylene oligomers via group IV-catalysed polymerization in very nonpolar media. Nat. Catal. 2019;2:236–242. [Google Scholar]
- Geyer R., Jambeck J.R., Law K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017;3:e1700782. doi: 10.1126/sciadv.1700782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau E., Broyer J.-P., Boisson C., Spitz R., Monteil V. Unusual activation by solvent of the ethylene free radical polymerization. Polym. Chem. 2011;2:2328. [Google Scholar]
- Guan Z. Control of polymer topology through transition-metal catalysis: synthesis of hyperbranched polymers by cobalt-mediated free radical polymerization. J. Am. Chem. Soc. 2002;124:5616–5617. doi: 10.1021/ja025609+. [DOI] [PubMed] [Google Scholar]
- Haider T., Shyshov O., Suraeva O., Lieberwirth I., von Delius M., Wurm F.R. Long-chain polyorthoesters as degradable polyethylene mimics. Macromolecules. 2019;52:2411–2420. doi: 10.1021/acs.macromol.9b00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider T.P., Volker C., Kramm J., Landfester K., Wurm F.R. Plastics of the future? The impact of biodegradable polymers on the environment and on society. Angew. Chem. Int. Ed. 2019;58:50–62. doi: 10.1002/anie.201805766. [DOI] [PubMed] [Google Scholar]
- Hedir G., Stubbs C., Aston P., Dove A.P., Gibson M.I. Synthesis of degradable poly(vinyl alcohol) by radical ring-opening copolymerization and ice recrystallization inhibition activity. ACS Macro Lett. 2017;6:1404–1408. doi: 10.1021/acsmacrolett.7b00905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedir G.G., Bell C.A., Ieong N.S., Chapman E., Collins I.R., O’Reilly R.K., Dove A.P. Functional degradable polymers by xanthate-mediated polymerization. Macromolecules. 2014;47:2847–2852. [Google Scholar]
- Hedir G.G., Bell C.A., O'Reilly R.K., Dove A.P. Functional degradable polymers by radical ring-opening copolymerization of MDO and vinyl bromobutanoate: synthesis, degradability and post-polymerization modification. Biomacromolecules. 2015;16:2049–2058. doi: 10.1021/acs.biomac.5b00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M.R., Guegain E., Tran J., Figg C.A., Turner A.C., Nicolas J., Sumerlin B.S. Radical ring-opening copolymerization of cyclic ketene acetals and maleimides affords homogeneous incorporation of degradable units. ACS Macro Lett. 2017;6:1071–1077. doi: 10.1021/acsmacrolett.7b00572. [DOI] [PubMed] [Google Scholar]
- Hill M.R., Kubo T., Goodrich S.L., Figg C.A., Sumerlin B.S. Alternating radical ring-opening polymerization of cyclic ketene acetals: access to tunable and functional polyester copolymers. Macromolecules. 2018;51:5079–5084. [Google Scholar]
- Hsu C.-S., Yang T.-Y., Peng C.-H. Vinyl acetate living radical polymerization mediated by cobalt porphyrins: kinetic–mechanistic studies. Polym. Chem. 2014;5:3867–3875. [Google Scholar]
- Jambeck J.R., Geyer R., Wilcox C., Siegler T.R., Perryman M., Andrady A., Narayan R., Law K.L. Plastic waste inputs from land into the ocean. Science. 2015;347:768–771. doi: 10.1126/science.1260352. [DOI] [PubMed] [Google Scholar]
- Kermagoret A., Debuigne A., Jerome C., Detrembleur C. Precision design of ethylene- and polar-monomer-based copolymers by organometallic-mediated radical polymerization. Nat. Chem. 2014;6:179–187. doi: 10.1038/nchem.1850. [DOI] [PubMed] [Google Scholar]
- Klein J., Briscoe B.J. Behavior of long molecules diffusing in solid polyethylene. Nature. 1977;266:43–44. [Google Scholar]
- Lebreton L.C.M., Van der Zwet J., Damsteeg J.W., Slat B., Andrady A., Reisser J. River plastic emissions to the world’s oceans. Nat. Commun. 2017;8:15611. doi: 10.1038/ncomms15611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., de Bruin B., Peng C.H., Fryd M., Wayland B.B. Exchange of organic radicals with organo-cobalt complexes formed in the living radical polymerization of vinyl acetate. J. Am. Chem. Soc. 2008;130:13373–13381. doi: 10.1021/ja804010h. [DOI] [PubMed] [Google Scholar]
- Liao C.-M., Hsu C.-C., Wang F.-S., Wayland B.B., Peng C.-H. Living radical polymerization of vinyl acetate and methyl acrylate mediated by Co(Salen*) complexes. Polym. Chem. 2013;4:3098. [Google Scholar]
- Lu Z., Fryd M., Wayland B.B. New life for living radical polymerization mediated by cobalt (II) metalloradicals. Macromolecules. 2004;37:2686–2687. [Google Scholar]
- Mattsson S., Dahlström M., Karlsson S. A mild hydrolysis of esters mediated by lithium salts. Tetrahedron Lett. 2007;48:2497–2499. [Google Scholar]
- Miao X.L., Zhu W., Zhang Z.B., Zhang W., Zhu X.L., Zhu J. Photo-induced cobalt-mediated radical polymerization of vinyl acetate. Polym. Chem. 2014;5:551–557. [Google Scholar]
- Modi P.J., Guillet J.E. The photochemistry of polymers containing anthrylmethyl vinyl ketone. J. Polym. Sci. Part A Polym. Chem. 1995;33:197–201. [Google Scholar]
- Peng C.H., Li S., Wayland B.B. Aspects of living radical polymerization mediated by cobalt porphyrin complexes. J. Chin. Chem. Soc. 2009;56:219–233. [Google Scholar]
- Savant D.M., Reddy D.V., McCord E.F., Rinaldi P.L. 2D NMR studies of poly (ethylene-co-vinyl acetate-co-carbon monoxide) Macromolecules. 2007;40:4199–4210. [Google Scholar]
- Scoponi M., Pradella F., Carassiti V. Photodegradable polyolefins. Photo-oxidation mechanisms of innovative polyolefin copolymers containing double bonds. Coord. Chem. Rev. 1993;125:219–230. [Google Scholar]
- Seema A., Liqun R. Polycaprolactone-based novel degradable ionomers by radical ring-opening polymerization of 2-methylene-1, 3-dioxepane. Macromolecules. 2009;42:1574–1579. [Google Scholar]
- Steinbach T., Wurm F.R. Poly(phosphoester)s: a new platform for degradable polymers. Angew. Chem. Int. Ed. 2015;54:6098–6108. doi: 10.1002/anie.201500147. [DOI] [PubMed] [Google Scholar]
- Tardy A., Honoré J.C., Tran J., Siri D., Delplace V., Bataille I., Letourneur D., Perrier J., Nicoletti C., Maresca M. Radical copolymerization of vinyl ethers and cyclic ketene acetals as a versatile platform to design functional polyesters. Angew. Chem. Int. Ed. 2017;56:16515–16520. doi: 10.1002/anie.201707043. [DOI] [PubMed] [Google Scholar]
- Tardy A., Nicolas J., Gigmes D., Lefay C., Guillaneuf Y. Radical ring-opening polymerization: scope, limitations, and application to (Bio)Degradable materials. Chem. Rev. 2017;117:1319–1406. doi: 10.1021/acs.chemrev.6b00319. [DOI] [PubMed] [Google Scholar]
- Undin J., Finne-Wistrand A., Albertsson A.C. Copolymerization of 2-methylene-1,3-dioxepane and glycidyl methacrylate, a well-defined and efficient process for achieving functionalized polyesters for covalent binding of bioactive molecules. Biomacromolecules. 2013;14:2095–2102. doi: 10.1021/bm4004783. [DOI] [PubMed] [Google Scholar]
- Undin J., Finne-Wistrand A., Albertsson A.C. Adjustable degradation properties and biocompatibility of amorphous and functional poly(ester-acrylate)-based materials. Biomacromolecules. 2014;15:2800–2807. doi: 10.1021/bm500689g. [DOI] [PubMed] [Google Scholar]
- Undin J., Illanes T., Finne-Wistrand A., Albertsson A.-C. Random introduction of degradable linkages into functional vinyl polymers by radical ring-opening polymerization, tailored for soft tissue engineering. Polym. Chem. 2012;3:1260–1266. [Google Scholar]
- Wang F.-S., Wang T.-F., Lu H.-H., Ao-Ieong W.-S., Wang J., Chen H.-L., Peng C.-H. Highly stretchable free-standing poly (acrylic acid)-block-poly (vinyl alcohol) films obtained from cobalt-mediated radical polymerization. Macromolecules. 2017;50:6054–6063. [Google Scholar]
- Wayland B.B., Peng C.H., Fu X.F., Lu Z., Fryd M. Degenerative transfer and reversible termination mechanisms for living radical polymerizations mediated by cobalt porphyrins. Macromolecules. 2006;39:8219–8222. [Google Scholar]
- Wolpers A., Bergerbit C., Ebeling B., d'Agosto F., Monteil V. Aromatic xanthates and dithiocarbamates for the polymerization of ethylene through reversible addition–fragmentation chain transfer (RAFT) Angew. Chem. Int. Ed. 2019;131:14433–14440. doi: 10.1002/anie.201905629. [DOI] [PubMed] [Google Scholar]
- Wu B., Lenz R.W. Synthesis, characterization, and hydrolytic degradation of copolymers of 2-methylene-1, 3-dioxepane with ethylene and with styrene. J. Environ. Polym. Degrad. 1998;6:23–29. [Google Scholar]
- Zhao Y., Zhang S., Wu Z., Liu X., Zhao X., Peng C.-H., Fu X. Visible-light-induced living radical polymerization (LRP) mediated by (salen) Co (II)/TPO at ambient temperature. Macromolecules. 2015;48:5132–5139. [Google Scholar]
- Zhao Y.G., Yu M.M., Zhang S.L., Wu Z.Q., Liu Y.C., Peng C.H., Fu X.F. A well-defined, versatile photoinitiator (salen)Co-CO2CH3 for visible light-initiated living/controlled radical polymerization. Chem. Sci. 2015;6:2979–2988. doi: 10.1039/c5sc00477b. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.