Abstract
Background and aims
A single nucleotide polymorphism of A118G (SNP; rs1799971) in the opioid receptor μ-1 (OPRM1) gene is a missense variant that influences the affinity of μ-opioid receptors. This study aimed to investigate the associations among the A118G polymorphism in the OPRM1 gene, psychiatric symptoms, and quantitative electroencephalography (qEEG) findings in patients with gambling disorder.
Methods
Fifty-five male patients with gambling disorder aged between 18 and 65 years old participated in the study. The A118G polymorphism was genotyped into the AA, GA, and GG groups by the polymerase chain reaction/restriction fragment length polymorphism method. Resting-state qEEG was recorded with the eyes closed, and the absolute power of the delta (1–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), and beta (12–30 Hz) frequency bands was analyzed. Psychiatric symptoms, including depression, anxiety, impulsivity and severity of gambling, were assessed by a self-rating scale.
Results
There were no significant differences in psychiatric symptoms among the three genotype groups (AA, GA, and GG). However, the frequency band power of qEEG showed significant differences among the three genotype groups. The absolute power of the beta and theta bands in the frontal lobe was higher in G allele carriers.
Discussion and conclusion
Based on the findings of this study, the polymorphism in the OPRM1 gene might affect the neurophysiological process in patients with gambling disorder.
Keywords: gambling disorder, μ-opioid receptors, A118G polymorphism, quantitative electroencephalography
Introduction
Although gambling is a popular leisure activity, pathological gambling is associated with impairments of social function, such as reduced quality of life, legal problems, bankruptcy, divorce, and incarceration (Argo & Black, 2004). The estimated past-year and lifetime prevalence of gambling disorder are approximately 0.2%–0.3% and 0.4%–1.0% of the general population, respectively (American Psychiatric Association [APA], 2013).
Pathological gambling was categorized into the Impulse-Control Disorders Not Elsewhere Classified section in Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) due to its impulsive characteristics (APA, 2000). However, recent neurobiological studies found similarities in brain structures and functions between subjects with pathological gambling and substance use disorder (Grant, Potenza, Weinstein, & Gorelick, 2010; Leeman & Potenza, 2012; Potenza, 2008). Thus, pathological gambling was renamed as gambling disorder and included in the Substance-Related and Addictive Disorders section in DSM-5 (APA, 2013). Substance use disorder is the most comorbid disorder with pathological gambling (Lorains, Cowlishaw, & Thomas, 2011).
Endogenous opioids such as β-endorphins and enkephalins are central neurotransmitters in addictive disorders. Opioids bind to μ-opioid receptors (MOPRs) in GABAergic interneurons in the ventral tegmental area, which is a reward processing pathway (Mague & Blendy, 2010). The binding of opioids results in the release of dopamine (DA) in the nucleus accumbens, which is associated with reinforcement and addiction (Di Chiara & Imperato, 1988). MOPRs are essential components of the opioid system, and a deficiency in these receptors is associated with altered behaviors and dependence (Matthes et al., 1996).
A single nucleotide polymorphism (SNP; rs1799971) in the opioid receptor μ-1 (OPRM1) gene is a missense variant that influences the affinity of MOPR. A transition from A to G on nucleotide 118 (A118G) encodes the substitution of an amino acid from asparagine to aspartate (ASP) in codon 40 (Bergen et al., 1997). A polymorphism in the OPRM1 gene was originally reported to influence the function of MOPRs, such as affinity of the receptor. The polymorphism causes a threefold increase in the binding affinity for β-endorphin (Bond et al., 1998). Furthermore, it alters the signal transduction cascade and receptor levels (Mura et al., 2013).
The OPRM1 gene is most widely studied in association with substance use disorder (Pfeifer et al., 2015; Rouvinen-Lagerström et al., 2013). The results of studies on the associations between the OPRM1 gene polymorphism and substance use disorder have been inconsistent. For instance, some studies reported significant associations between alcohol dependence and the A118G polymorphism (Bart et al., 2005; Nishizawa et al., 2006; Rommelspacher, Smolka, Schmidt, Samochowiec, & Hoehe, 2001), whereas other studies did not report associations (Bergen et al., 1997; Loh, Fann, Chang, Chang, & Cheng, 2004). A recent meta-analysis reported did not report a strong association between the A118G polymorphism and alcohol dependence due to limitations of heterogeneities and small sample sizes (Kong et al., 2017).
Several studies have reported the associations between the rs1799971 SNP and clinical outcomes in subjects with a heroin addiction. The polymorphism was associated with long-term abstinence from heroin without treatment (Levran et al., 2017) and consequences related to heroin use, such as unexpected reactions or overdose (Woodcock, Lundahl, Burmeister, & Greenwald, 2015). Although Arias, Feinn, and Kranzler (2006) did not identify an association between the polymorphism in the OPRM1 gene and the risk of general substance dependence, a recent meta-analysis showed that no relationship was observed in African or Caucasian populations, but was observed in Asian populations (Haerian & Haerian, 2013). Similarly, the polymorphism affects other substance addictions, such as nicotine addiction (Verhagen, Kleinjan, & Engels, 2012).
Although opioid antagonists, such as nalmefene and naltrexone, have been studied for the treatment of gambling disorder (Grant et al., 2006), there is no study on the direct association between the OPRM1 gene polymorphism and gambling disorder. A study of gambling disorder treated with naltrexone investigated the treatment effect with the interaction of the A118G polymorphism and reported that patients with the AA genotype showed increased emotional well-being (Kovanen et al., 2016).
Electroencephalography (EEG) is an electrophysiological monitoring tool used to assess the electrical activity of the brain, and quantitative electroencephalography (qEEG) includes an analysis of the power spectra of the delta (1–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), and beta (12–30 Hz) frequency bands. Most research on the EEG correlates of gambling has focused on reward sensitivities and decision-making in gambling tasks and suggests that the feedback negativity event-related potential is sensitive to outcome expectation (Houston & Ceballos, 2013).
However, no study has addressed the association between resting-state qEEG and the A118G SNP in patients with gambling disorder. Thus, this study aimed to investigate the associations among psychopathology, band power spectrum of resting-state qEEG, and OPRM1 gene polymorphism in gambling disorder.
Methods
Study setting, data sources, and sample
Individuals who visited the gambling disorder clinic in a university hospital, South Korea, were considered for inclusion in the study. The inclusion criteria were diagnosis of gambling disorder according to DSM-5 criteria, male gender, and age between 18 and 65 years old. Subjects were excluded if any of the following applied: a diagnosis of substance use disorder other than nicotine and caffeine based on DSM-5; use of psychotropic medications over the last year; and the presence of a physical, mental disorder, or neurological disorder. Based on the inclusion and exclusion criteria, 55 male subjects (age: 38.42 ± 11.59 years) were enrolled in this study. None of the participants were taking any medications, and all had completed at least 12 years of education (14.65 ± 1.89 years).
Measures
Psychosocial variables
Psychosocial variables were assessed with the following validated self-rating scales: Beck Depression Inventory for depression, Beck Anxiety Inventory for anxiety, Barratt Impulsiveness Scales for impulsivity, Lubben Social Network Scale for social networks, Wender–Utah Attention Deficit/Hyperactivity Disorder Rating Scale for childhood attention-deficit/hyperactivity symptoms, the 9-item Problem Gambling Severity Index from the Canandian Problem Gambling Index, and the Gambling Symptom Assessment Scale for the severity of gambling disorder.
DNA analysis
Approximately 10 ml of ethylenediaminetetraacetic acid-treated venous blood was obtained for DNA extraction from each subject. Genomic DNA was extracted from blood samples by standard methods (Lahiri &Schnabel, 1993). The A118G polymorphism was genotyped using the polymerase chain reaction/restriction fragment length polymorphism method described by Gelernter, Kranzler, and Cubells (1999).
EEG recording and preprocessing
EEG recordings were performed using a SynAmps2 direct current (DC) amplifier and a 10–20 layout 64-channel Quick-Cap electrode-placement system (Neuroscan Inc., NC, USA). EEG data were digitally recorded from 19 gold cup electrodes placed according to the international 10–20 system. Impedances were maintained below 5 kΩ, and the sampling rate was 1,000 Hz. We used the linked mastoid reference and two additional bipolar electrodes to measure horizontal and vertical eye movements. During the recording, each participant laid in a semidarkened, electrically shielded, sound-attenuated room. A resting EEG was recorded after 3 min having the participant’s eyes closed.
We used MATLAB 7.0.1 (Math Works, Natick, MA, USA) and the EEGLAB toolbox (Delorme & Makeig, 2004) to preprocess and analyze EEG recordings. First, EEG data were downsampled to 250 Hz. Next, EEG data were detrended and mean-subtracted to remove the DC component. A 1-Hz high-pass filter and 60-Hz notch filter were applied to remove the eye and electrical noise. Next, independent component analysis (ICA) was performed to remove well-defined sources of artifacts. ICA has been demonstrated to reliably isolate artifacts caused by eye and muscle movements and heart noise (Jung et al., 2000). Finally, clinical psychiatrists and EEG experts visually inspected the corrected EEGs. For the analysis, we selected more than 2 min of artifact-free EEG readings from 3-min recordings.
EEG analysis
Four frequency bands were defined for further analysis, delta (1–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), and beta (12–30 Hz). We investigated the power spectra of the EEG data from each subject using the short-time Fourier transform “spectrogram.m” function from the Signal Processing Toolbox in MATLAB. A time window of 1,000 ms with an 800-ms overlap and Hamming window were used for the spectral analysis. Outliers that were far from the spectral value distribution of each frequency band at the 0.05 significance level were removed. Finally, the absolute power was averaged over all of the time windows and frequency bands for further analysis.
Statistical analyses
We divided OPRM1 A118G into the AG, GG, and AA genotype groups. Analysis of variance (ANOVA) across the genotype was used to test group differences in demographic, clinical characteristics, and EEG data. The homogeneity of slopes between the groups was first assessed using Levene’s test to follow-up tests at individual electrodes. If the slopes were homogeneous, a second step was performed using one-way ANOVA to compare the activity recorded at each individual electrode among the three groups. Statistical significance was defined as p < .05. We used the false discovery rate (FDR) correction, in which p values were multiplied by the number of comparisons, to control false positives in multiple comparisons. We used Bonferroni-corrected post-hoc comparisons for three groups to determine specific group differences (p < .0167). All data were analyzed using Statistical Package for the Social Sciences (SPSS) statistical software, version 18.0 (SPSS Inc., Chicago, IL, USA).
Ethics
This study was approved by the Institutional Review Board of the Eulji Medical Center (Seoul, South Korea; NSU-161220-01) and was performed in accordance with the Declaration of Helsinki. All participants submitted written informed consent after receiving a complete description of the study and were not compensated for their participation in the study.
Results
Demographic and clinical characteristics
The total sample comprised 55 male individuals with gambling disorder [mean age (SD) = 38.42 (11.59) years]. Twenty-three subjects had the AA genotype, 20 had the GA genotype, and 12 had the GG genotype. No deviation from Hardy–Weinberg equilibrium was observed in this study population (χ2 = 0.03, p = .99). There were no differences in demographic data and clinical characteristics between the genotype groups. Demographic and clinical characteristics are summarized in Table 1.
Table 1.
Demographic and clinical characteristics of subjects
| Subject (n) | AA | GA | GG | F | p value |
|---|---|---|---|---|---|
| 23 | 20 | 12 | |||
| Age (years) | 37.17 ± 10.51 | 40.00 ± 13.14 | 38.17 ± 11.50 | 0.313 | .732 |
| Education (year) | 14.26 ± 2.03 | 14.70 ± 2.08 | 15.33 ± 0.98 | 1.296 | .282 |
| BDI | 15.30 ± 10.20 | 19.45 ± 9.47 | 14.50 ± 7.01 | 1.456 | .243 |
| BAI | 12.78 ± 9.47 | 12.50 ± 11.21 | 12.33 ± 7.96 | 0.009 | .991 |
| BIS | 58.43 ± 7.46 | 55.10 ± 9.93 | 54.75 ± 6.37 | 1.186 | .313 |
| LSNS | 24.96 ± 4.56 | 24.25 ± 5.86 | 25.00 ± 6.25 | 0.112 | .894 |
| WURS | 30.57 ± 17.11 | 29.90 ± 18.08 | 29.42 ± 8.61 | 0.022 | .978 |
| CPGI-PGIS | 18.30 ± 7.02 | 19.50 ± 5.83 | 18.92 ± 5.37 | 0.195 | .824 |
| GSAS | 23.74 ± 14.32 | 26.75 ± 9.56 | 16.67 ± 10.46 | 2.688 | .077 |
Note. Analysis of variance and post hoc test were used. Data are given as mean ± standard deviation. BDI: Beck Depression Inventory; BAI: Beck Anxiety Inventory; BIS: The Korean version of Barratt Impulsiveness Scale; LSNS: Lubben Social Network Scale; WURS: Wender–Utah Rating Scale; CPGI-PGIS: Canadian Problem Gambling Index–Problem Gambling Severity Index; GSAS: Gambling Symptom Assessment Scale.
QEEG activity
Theta and delta absolute power
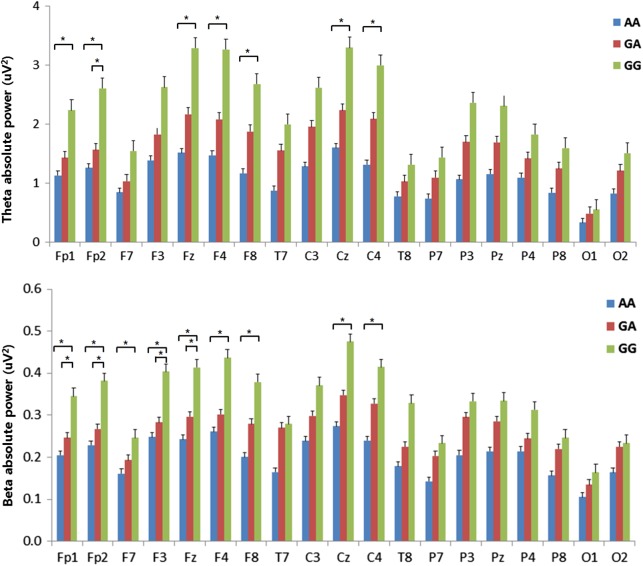
Theta absolute power exhibited significant differences among the genotypes. After the Bonferroni correction of post-hoc comparisons, the AA genotype showed lower power in the theta frequency than the GG genotype at 7 of the electrodes (Fp1, Fp2, Fz, F4, F8, Cz, and C4). There was a significant difference at the Fp2 electrode between the GA and GG genotypes (GA < GG), but there were no significant differences between the AA and GA genotypes. All significant differences were FDR-corrected (Figure 1). Delta absolute power also exhibited significant differences in a focal region of F8.
Figure 1.
Absolute EEG power in theta and beta bands during the resting-state condition. The horizontal bars represent standard errors. *Significant difference in the post-hoc test with the Bonferroni correction (p < .0167)
Beta absolute power
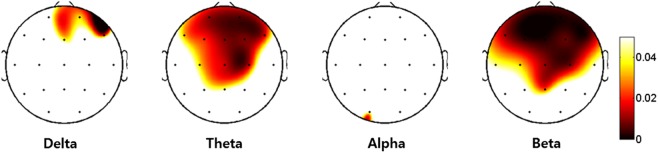
Beta absolute power showed significant differences among the genotypes. After the Bonferroni correction of the post-hoc comparisons, the AA genotype showed lower power in the beta frequency than the GG genotype at 9 of the electrodes (Fp1, Fp2, F7, F3, Fz, F4, F8, Cz, and C4). Four of the electrodes (Fp1, Fp2, F3, and Fz) exhibited significant differences between the GA and GG genotypes, but there were no significant differences between the AA and GA genotypes. All significant differences were FDR-corrected (Figure 1). Figure 2 shows the scalp topographies of the three groups in terms of the absolute power in each band.
Figure 2.
The topographic maps representing the probability of analysis of variance among groups. Scale show μV2 for absolute power. A colored area means an increase of difference in absolute powers
Discussion
This study investigated the associations between the power spectrum of EEG frequency bands and the μ-opioid polymorphism A118G in patients with gambling disorder. All of the participants in this study were Korean (Asian) and 58.2% of participants carried the 118G allele (n = 32). This finding is in consistent with a previous study in which the 118G (ASP40) allele showed the lowest frequencies in Africans (<5%) and the highest frequencies (25%–45%) in Asians (Arias et al., 2006). Moreover, other studies analyzing Korean alcohol-dependent patients reported a percentage of carriers of 118G allele ranging from 54% to 67% (Kim, Kim, Song, et al., 2004; Kim, Kim, Kang, et al., 2004).
The psychiatric symptoms assessed by self-rating scales, such as depression, anxiety, impulsivity, and severity of gambling, were not significantly different among the three OPRM1 polymorphism groups. Although there are no studies on the direct association of the psychopathology of gambling disorder and A118G polymorphism, these results seem consistent with previous studies that reported no association between the OPRM1 gene polymorphism and substance dependence, including heroin and alcohol (Arias et al., 2006; Kong et al., 2017).
In contrast to psychiatric symptoms, the frequency band power of qEEG showed significant differences among the three groups. In carriers of the 118G allele, the absolute power of the beta and theta bands in the frontal lobe was higher in patients without the 118G allele. These results are interesting and suggest that the polymorphism in the OPRM1 gene might alter the neurophysiological processes of patients with gambling disorder, although the subjective psychiatric symptoms do not differ.
Increased absolute power of the theta and delta bands (slow wave) in the frontal area is a common finding in patients with psychiatric disorders, such as attention-deficit/hyperactivity disorder (ADHD), intermittent explosive disorder, and substance use disorders, which are characterized by impulsivity (Hanafiah, Taib, & Hamid, 2010; Kamarajan & Porjesz, 2012; Rangaswamy et al., 2003; Tye, Rijsdijk, & McLoughlin, 2014). Increased theta power might be associated with dopaminergic function. Catechol-O-methyl-transferase (COMT) is an enzyme that metabolizes DA, and the COMT gene Val158Met SNP was reportedly associated with theta power (Lee et al., 2011). The COMT gene Val variant degrades DA at up to four times the rate of the Met variant and results in reduced synaptic DA. According to Lee et al. (2011), healthy females with the Val variant showed increased theta power in the frontal and central regions. The binding of opioids to MOPRs induces the release of DA in the reward circuit (Heinz et al., 2009). Furthermore, the OPRM1 polymorphism alters addictive behavior and striatal DA concentrations in a mouse model (Zhang et al., 2015), and changes in the DA pathway were identified with increased theta power in the frontal cortex (Jang, Kim, Kim, & Lee, 2009). Thus, the increased theta power observed in patients carrying the G allele suggests that differences in the binding affinity of the MOPR caused by the polymorphism in the OPRM1 gene might be associated with altered theta power related to the dopaminergic system.
In this study, patients carrying the G allele also showed higher absolute power in the beta band than patients without the G allele. Despite some studies regarding the EEG correlates of gambling behavior using a “gambling task,” few studies have addressed EEG band power among pathologic gamblers (Houston & Ceballos, 2013). However, across the majority of “impulsive spectrum disorders” similar to gambling disorder, including substance abuse disorders, conduct disorder, ADHD, antisocial personality disorder, and eating disorder, the major EEG finding is excessive beta power in resting status (Kamarajan & Porjesz, 2012). Increased power of beta bands may reflect a hyperexcitable state that is produced by an excitation–inhibition imbalance mediated by GABAergic synaptic potentials (Faulkner, Traub, & Whittington, 1999; Rangaswamy et al., 2002).
For instance, increased beta bands are also a characteristic feature in alcoholism (Kamarajan & Porjesz, 2012). Previous studies have indicated decreased levels of GABA-benzodiazepine receptors in alcoholics (Abi-Dargham et al., 1998; Lingford-Hughes et al., 1998). The associations between the GABA receptor gene and the EEG beta band have been established (Porjesz et al., 2002). GABAergic interneurons maintain inhibition of dopaminergic neurons (Peciña, Love, Stohler, Goldman, & Zubieta, 2015). The binding of opioids to MOPRs decreases this inhibition and results in the release of DA from the GABAergic interneuron (Johnson & North, 1992; Mague & Blendy, 2010). This phasic release of DA is considered to contribute to substance dependence (Wise & Bozarth, 1985). An increased beta band in patients with the G allele indicates that differences in the binding affinity of the MOPR according to the OPRM1 gene polymorphism might be associated with the GABAergic system. Consequently, together with differences in the theta band, the polymorphism in the OPRM1 gene polymorphism might be associated with alterations in the DA system.
This study has several limitations that should be noted. First, this study did not include healthy controls, which limit the comparison between the patients and controls as well as a generalized interpretation of our findings for normal populations. Our small sample size is another shortcoming of this study. Future studies including healthy controls and a larger sample are needed to confirm the present findings. Second, this study used only a self-rating scale to assess psychiatric symptoms and the severity of pathological gambling. Pathological gamblers often have poor insight, which might cause incorrect reports of psychiatric symptoms or the severity of gambling. Potentially, the lack of significant differences in depression, anxiety, impulsivity and severity of gambling among the three groups in this study resulted from inaccurate self-reporting. Thus, reports from multiple informants, including participants’ families, could improve the reliability of the assessment for the psychiatric symptoms of participants. In addition, some neuropsychological tests could provide more accurate information about a patient’s cognition and impulsivity. Future studies, including multi-informant reports and neuropsychological tests, could reveal more subtle differences in psychological aspects related to the OPRM1 gene and qEEG.
Despite some limitations, to our knowledge, this is the first study to investigate the association between OPRM1 gene polymorphism and qEEG frequency band power in pathological gambling. In this study, the absolute power of the beta and theta bands in the frontal and central regions was increased in patients carrying the G allele compared with patients without the G allele, and the increased power was not closely associated with psychiatric symptoms or the severity of gambling. Our findings will help to extend the understanding of the neurophysiologic changes related to OPRM1 gene polymorphism.
Funding Statement
Funding sources: This work was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health and Welfare, South Korea (no. A120157).
Authors’ contribution
S-WC, JL, and JWK contributed to conceptualization and data curation. KMK, JL, and JWK contributed to formal analysis. KMK and S-WC contributed to writing of original draft and contributed equally to this work. KMK, S-WC, JL, JWK, and DK contributed to writing of review and editing.
Conflict of interest
The authors have no potential conflicts of interest to disclose.
References
- Abi-Dargham A., Krystal J. H., Anjilvel S., Scanley B. E., Zoghbi S., Baldwin R. M., Rajeevan N., Ellis S., Petrakis I. L., Seibyl J. P., Charney D. S., Laruelle M., Innis R. B. (1998). Alterations of benzodiazepine receptors in type II alcoholic subjects measured with SPECT and [123I] iomazenil. American Journal of Psychiatry, 155(11), 1550–1555. doi: 10.1176/ajp.155.11.1550 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association [APA]. (2000). DSM-IV-TR: Diagnostic and statistical manual of mental disorders (text rev., Vol. 75, pp. 78–85). Washington, DC: American Psychiatric Association. [Google Scholar]
- American Psychiatric Association [APA]. (2013). Diagnostic and statistical manual of mental disorders (DSM-5®). Washington, DC: American Psychiatric Association. [Google Scholar]
- Argo T. R., Black D. W. (2004). Clinical characteristics. In Grant J. E., Potenza M. N. (Eds.), Pathological gambling: A clinical guide to treatment (pp. 39–53). Arlington, VA: American Psychiatric Publishing, Inc. [Google Scholar]
- Arias A., Feinn R., Kranzler H. R. (2006). Association of an Asn40Asp (A118G) polymorphism in the μ-opioid receptor gene with substance dependence: A meta-analysis. Drug & Alcohol Dependence, 83(3), 262–268. doi: 10.1016/j.drugalcdep.2005.11.024 [DOI] [PubMed] [Google Scholar]
- Bart G., Kreek M. J., Ott J., LaForge K. S., Proudnikov D., Pollak L., Heilig M. (2005). Increased attributable risk related to a functional μ-opioid receptor gene polymorphism in association with alcohol dependence in central Sweden. Neuropsychopharmacology, 30(2), 417–422. doi: 10.1038/sj.npp.1300598 [DOI] [PubMed] [Google Scholar]
- Bergen A., Kokoszka J., Peterson R., Long J., Virkkunen M., Linnoila M., Goldman D. (1997). μ opioid receptor gene variants: Lack of association with alcohol dependence. Molecular Psychiatry, 2(6), 490–494. doi: 10.1038/sj.mp.4000331 [DOI] [PubMed] [Google Scholar]
- Bond C., LaForge K. S., Tian M., Melia D., Zhang S., Borg L., Gong J., Schluger J., Strong J. A., Leal S. M., Tischfield J. A., Kreek M. J., Yu L. (1998). Single-nucleotide polymorphism in the human mu opioid receptor gene alters β-endorphin binding and activity: Possible implications for opiate addiction. Proceedings of the National Academy of Sciences of the United States of America, 95(16), 9608–9613. doi: 10.1073/pnas.95.16.9608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A., Makeig S. (2004). EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21. doi: 10.1016/j.jneumeth.2003.10.009 [DOI] [PubMed] [Google Scholar]
- Di Chiara G., Imperato A. (1988). Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences of the United States of America, 85(14), 5274–5278. doi: 10.1073/pnas.85.14.5274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner H., Traub R., Whittington M. (1999). Anaesthetic/amnesic agents disrupt beta frequency oscillations associated with potentiation of excitatory synaptic potentials in the rat hippocampal slice. British Journal of Pharmacology, 128(8), 1813–1825. doi: 10.1038/sj.bjp.0702948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelernter J., Kranzler H., Cubells J. (1999). Genetics of two μ opioid receptor gene (OPRM1) exon I polymorphisms: Population studies, and allele frequencies in alcohol-and drug-dependent subjects. Molecular Psychiatry, 4(5), 476–483. doi: 10.1038/sj.mp.4000556 [DOI] [PubMed] [Google Scholar]
- Grant J. E., Potenza M. N., Hollander E., Cunningham-Williams R., Nurminen T., Smits G., Kallio A. (2006). Multicenter investigation of the opioid antagonist nalmefene in the treatment of pathological gambling. American Journal of Psychiatry, 163(2), 303–312. doi: 10.1176/appi.ajp.163.2.303 [DOI] [PubMed] [Google Scholar]
- Grant J. E., Potenza M. N., Weinstein A., Gorelick D. A. (2010). Introduction to behavioral addictions. The American Journal of Drug and Alcohol Abuse, 36(5), 233–241. doi: 10.3109/00952990.2010.491884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haerian B. S., Haerian M. S. (2013). OPRM1 rs1799971 polymorphism and opioid dependence: Evidence from a meta-analysis. Pharmacogenomics, 14(7), 813–824. doi: 10.2217/pgs.13.57 [DOI] [PubMed] [Google Scholar]
- Hanafiah Z. M., Taib M. N., Hamid N. H. A. (2010). EEG pattern of smokers for theta, alpha and beta band frequencies. Paper presented at the 2010 IEEE Student Conference on Research and Development (SCOReD), Putrajaya, Malaysia. [Google Scholar]
- Heinz A., Beck A., Wrase J., Mohr J., Obermayer K., Gallinat J., Puls I. (2009). Neurotransmitter systems in alcohol dependence. Pharmacopsychiatry, 42(Suppl. 1), S95–S101. doi: 10.1055/s-0029-1214395 [DOI] [PubMed] [Google Scholar]
- Houston R. J., Ceballos N. A. (2013). Chapter 38: Human neurophysiology: EEG and quantitative EEG in addiction research. In Miller P. (Ed.), Biological research on addiction of comprehensive addictive behaviors and disorders. San Diego, CA: Elsevier Inc. [Google Scholar]
- Jang H. S., Kim J. Y., Kim S. H., Lee M. G. (2009). Role of dopamine receptors on electroencephalographic changes produced by repetitive apomorphine treatments in rats. The Korean Journal of Physiology and Pharmacology, 13(3), 147–151. doi: 10.4196/kjpp.2009.13.3.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S., North R. (1992). Opioids excite dopamine neurons by hyperpolarization of local interneurons. Journal of Neuroscience, 12(2), 483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung T.-P., Makeig S., Humphries C., Lee T.-W., Mckeown M. J., Iragui V., Sejnowski T. J. (2000). Removing electroencephalographic artifacts by blind source separation. Psychophysiology, 37(2), 163–178. doi: 10.1111/1469-8986.3720163 [DOI] [PubMed] [Google Scholar]
- Kamarajan C., Porjesz B. (2012). Brain waves in impulsivity spectrum disorders. In Cyders M. A. (Ed.), Psychology of impulsivity (pp. 20–93). Hauppauge, NY: Nova Science Publishers. [Google Scholar]
- Kim S. A., Kim J.-W., Song J.-Y., Park S., Lee H. J., Chung J.-H. (2004a). Association of polymorphisms in nicotinic acetylcholine receptor α4 subunit gene (CHRNA4), μ-opioid receptor gene (OPRM1), and ethanol-metabolizing enzyme genes with alcoholism in Korean patients. Alcohol, 34(2–3), 115–120. doi: 10.1016/j.alcohol.2004.06.004 [DOI] [PubMed] [Google Scholar]
- Kim S. G., Kim C. M., Kang D. H., Kim Y. J., Byun W. T., Kim S. Y., Park J. M., Kim M. J., Oslin D. W. (2004b). Association of functional opioid receptor genotypes with alcohol dependence in Koreans. Alcoholism: Clinical and Experimental Research, 28(7), 986–990. doi: 10.1097/01.ALC.0000130803.62768.AB [DOI] [PubMed] [Google Scholar]
- Kong X., Deng H., Gong S., Alston T., Kong Y., Wang J. (2017). Lack of associations of the opioid receptor mu 1 (OPRM1) A118G polymorphism (rs1799971) with alcohol dependence: Review and meta-analysis of retrospective controlled studies. BMC Medical Genetics, 18(1), 120. doi: 10.1186/s12881-017-0478-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovanen L., Basnet S., Castren S., Pankakoski M., Saarikoski S. T., Partonen T., Alho H., Lahti T. (2016). A randomised, double-blind, placebo-controlled trial of as-needed naltrexone in the treatment of pathological gambling. European Addiction Research, 22(2), 70–79. doi: 10.1159/000435876 [DOI] [PubMed] [Google Scholar]
- Lahiri D. K., Schnabel B. (1993). DNA isolation by a rapid method from human blood samples: Effects of MgCl 2, EDTA, storage time, and temperature on DNA yield and quality. Biochemical Genetics, 31(7–8), 321–328. doi: 10.1007/BF00553174 [DOI] [PubMed] [Google Scholar]
- Lee T.-W., Younger W., Hong C.-J., Tsai S.-J., Wu H.-C., Chen T.-J. (2011). The effects of catechol-O-methyl-transferase polymorphism Val158Met on functional connectivity in healthy young females: A resting EEG study. Brain Research, 1377, 21–31. doi: 10.1016/j.brainres.2010.12.073 [DOI] [PubMed] [Google Scholar]
- Leeman R. F., Potenza M. N. (2012). Similarities and differences between pathological gambling and substance use disorders: A focus on impulsivity and compulsivity. Psychopharmacology, 219(2), 469–490. doi: 10.1007/s00213-011-2550-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levran O., Peles E., Randesi M., da Rosa J., Adelson M., Kreek M. (2017). The μ-opioid receptor nonsynonymous variant 118A>G is associated with prolonged abstinence from heroin without agonist treatment. Pharmacogenomics, 18(15), 1387–1391. doi: 10.2217/pgs-2017-0092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingford-Hughes A. R., Acton P., Gacinovic S., Suckling J., Busatto G., Boddington S., Bullmore E., Woodruff P. W., Costa D. C., Pilowsky L. S., Ell P. J., Marshall E. J., Kerwin R. W. (1998). Reduced levels of GABA-benzodiazepine receptor in alcohol dependency in the absence of grey matter atrophy. The British Journal of Psychiatry, 173(2), 116–122. doi: 10.1192/bjp.173.2.116 [DOI] [PubMed] [Google Scholar]
- Loh E. W., Fann C. S., Chang Y. T., Chang C. J., Cheng A. T. (2004). Endogenous opioid receptor genes and alcohol dependence among Taiwanese Han. Alcoholism: Clinical and Experimental Research, 28(1), 15–19. doi: 10.1097/01.ALC.0000106303.41755.B8 [DOI] [PubMed] [Google Scholar]
- Lorains F. K., Cowlishaw S., Thomas S. A. (2011). Prevalence of comorbid disorders in problem and pathological gambling: Systematic review and meta-analysis of population surveys. Addiction, 106(3), 490–498. doi: 10.1111/j.1360-0443.2010.03300.x [DOI] [PubMed] [Google Scholar]
- Mague S. D., Blendy J. A. (2010). OPRM1 SNP (A118G): Involvement in disease development, treatment response, and animal models. Drug & Alcohol Dependence, 108(3), 172–182. doi: 10.1016/j.drugalcdep.2009.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthes H. W., Maldonado R., Simonin F., Valverde O., Slowe S., Kitchen I., Befort K., Dierich A., Le Meur M., Dollé P., Tzavara E., Hanoune J., Roques B. P., Kieffer B. L. (1996). Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the μ-opioid-receptor gene. Nature, 383(6603), 819–823. doi: 10.1038/383819a0 [DOI] [PubMed] [Google Scholar]
- Mura E., Govoni S., Racchi M., Carossa V., Ranzani G. N., Allegri M., van Schaik R. H. (2013). Consequences of the 118A>G polymorphism in the OPRM1 gene: Translation from bench to bedside? Journal of Pain Research, 6, 331. doi: 10.2147/JPR.S42040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa D., Han W., Hasegawa J., Ishida T., Numata Y., Sato T., Kawai A., Ikeda K. (2006). Association of μ-opioid receptor gene polymorphism A118G with alcohol dependence in a Japanese population. Neuropsychobiology, 53(3), 137–141. doi: 10.1159/000093099 [DOI] [PubMed] [Google Scholar]
- Peciña M., Love T., Stohler C. S., Goldman D., Zubieta J.-K. (2015). Effects of the Mu opioid receptor polymorphism (OPRM1 A118G) on pain regulation, placebo effects and associated personality trait measures. Neuropsychopharmacology, 40(4), 957–965. doi: 10.1038/npp.2014.272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer P., Sariyar M., Eggermann T., Zerres K., Vernaleken I., Tüscher O., Fehr C. (2015). Alcohol consumption in healthy OPRM1 G allele carriers and its association with impulsive behavior. Alcohol and Alcoholism, 50(4), 379–384. doi: 10.1093/alcalc [DOI] [PubMed] [Google Scholar]
- Porjesz B., Almasy L., Edenberg H. J., Wang K., Chorlian D. B., Foroud T., Goate A., Rice J. P., O’Connor S. J., Rohrbaugh J., Kuperman S., Bauer L. O., Crowe R. R., Schuckit M. A., Hesselbrock V., Conneally P. M., Tischfield J. A., Li T. K., Reich T., Begleiter H. (2002). Linkage disequilibrium between the beta frequency of the human EEG and a GABAA receptor gene locus. Proceedings of the National Academy of Sciences of the United States of America, 99(6), 3729–3733. doi: 10.1073/pnas.052716399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza M. N. (2008). The neurobiology of pathological gambling and drug addiction: An overview and new findings. Philosophical Transactions of the Royal Society B: Biological Sciences, 363(1507), 3181–3189. doi: 10.1098/rstb.2008.0100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangaswamy M., Porjesz B., Chorlian D. B., Choi K., Jones K. A., Wang K., Rohrbaugh J., O’Connor S., Kuperman S., Reich T., Begleiter H. (2003). Theta power in the EEG of alcoholics. Alcoholism: Clinical and Experimental Research, 27(4), 607–615. doi: 10.1111/j.1530-0277.2003.tb04397.x [DOI] [PubMed] [Google Scholar]
- Rangaswamy M., Porjesz B., Chorlian D. B., Wang K., Jones K. A., Bauer L. O., Rohrbaugh J., O’Connor S. J., Kuperman S., Reich T., Begleiter H. (2002). Beta power in the EEG of alcoholics. Biological Psychiatry, 52(8), 831–842. doi: 10.1016/S0006-3223(02)01362-8 [DOI] [PubMed] [Google Scholar]
- Rommelspacher H., Smolka M., Schmidt L. G., Samochowiec J., Hoehe M. R. (2001). Genetic analysis of the μ-opioid receptor in alcohol-dependent individuals. Alcohol, 24(2), 129–135. doi: 10.1016/S0741-8329(01)00139-2 [DOI] [PubMed] [Google Scholar]
- Rouvinen-Lagerström N., Lahti J., Alho H., Kovanen L., Aalto M., Partonen T., Silander K., Sinclair D., Räikkönen K., Eriksson J. G., Palotie A., Koskinen S., Saarikoski S. T. (2013). μ-Opioid receptor gene (OPRM1) polymorphism A118G: Lack of association in Finnish populations with alcohol dependence or alcohol consumption. Alcohol and Alcoholism, 48(5), 519–525. doi: 10.1093/alcalc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye C., Rijsdijk F., McLoughlin G. (2014). Genetic overlap between ADHD symptoms and EEG theta power. Brain and Cognition, 87, 168–172. doi: 10.1016/j.bandc.2014.03.010 [DOI] [PubMed] [Google Scholar]
- Verhagen M., Kleinjan M., Engels R. C. (2012). A systematic review of the A118G (Asn40Asp) variant of OPRM1 in relation to smoking initiation, nicotine dependence and smoking cessation. Pharmacogenomics, 13(8), 917–933. doi: 10.2217/pgs.12.76 [DOI] [PubMed] [Google Scholar]
- Wise R., Bozarth M. (1985). Brain mechanisms of drug reward and euphoria. Psychiatric Medicine, 3(4), 445–460. [PubMed] [Google Scholar]
- Woodcock E. A., Lundahl L. H., Burmeister M., Greenwald M. K. (2015). Functional mu opioid receptor polymorphism (OPRM1 A118G) associated with heroin use outcomes in Caucasian males: A pilot study. The American Journal on Addictions, 24(4), 329–335. doi: 10.1111/ajad.12187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Picetti R., Butelman E. R., Ho A., Blendy J. A., Kreek M. J. (2015). Mouse model of the OPRM1 (A118G) polymorphism: Differential heroin self-administration behavior compared with wild-type mice. Neuropsychopharmacology, 40(5), 1091–1100. doi: 10.1038/npp.2014.286 [DOI] [PMC free article] [PubMed] [Google Scholar]