Abstract
Rho-associated protein kinase (ROCK) signaling correlates with cell shape, with decreased cell spreading accompanied by decreased ROCK activity. However, how cell shape and ROCK activity impact the chondrogenesis of mesenchymal stem cells (MSCs) remains inconclusive. Here we examine the effects of ROCK inhibition on human MSC chondrogenesis in four different culture models, including three-dimensional (3D) microribbon (μRB) scaffolds, two-dimensional hydrogel (2D-HG) substrates, 3D hydrogels (3D-HGs), and pellet. For each culture model involving biomaterials, four polymers were compared, including gelatin, chondroitin sulfate, hyaluronic acid, and polyethylene glycol. ROCK inhibition decreased MSC chondrogenesis in μRB model, enhanced chondrogenesis in pellet, and had minimal effect in 2D-HG or 3D-HG models. Furthermore, we demonstrate that MSC chondrogenesis cannot be predicted using ROCK signaling alone. While varying biomaterial compositions can impact the amount or phenotype of resulting cartilage, varying biomaterials did not change the chondrogenic response to ROCK inhibition within each culture model. Regardless of culture model or ROCK expression, increased cartilage formation was always accompanied by enhanced N-cadherin expression and production, suggesting that N-cadherin is a robust marker to select culture conditions that promote chondrogenesis. Together, the results from this study may be used to enhance MSC-based cartilage regeneration in different culture models.
Impact Statement
Here we assessed the effects of Rho-associated protein kinase (ROCK) inhibition on mesenchymal stem cell (MSC) chondrogenesis in different culture models, including three-dimensional (3D) microribbon scaffolds, two-dimensional hydrogel substrates, 3D hydrogels, and pellet culture. Our results demonstrate that effects of ROCK inhibition on MSC chondrogenesis differ substantially depending on culture models. Furthermore, MSC chondrogenesis cannot be predicted using ROCK signaling alone. The results from this study fill in a gap of knowledge in the correlation between ROCK signaling and MSC chondrogenesis, which may be used to enhance MSC-based cartilage regeneration in different culture models.
Keywords: Y27632, ROCK inhibition, cartilage, mesenchymal stem cell, culture model
Introduction
The initial stage of cartilage development is mesenchymal condensation, during which undifferentiated mesenchymal progenitor cells begin to adopt rounded morphologies, aggregate together, and upregulate chondrogenic genes.1,2 Cell shape correlates with rho-associated protein kinase (ROCK) signaling, as round cells exhibit lower ROCK activity than spread cells.3,4 Chondrocytes, the resident cell type in articular cartilage, are characterized by a round cell shape. Previous work has demonstrated that the phenotype of chondrocytes is highly dependent on cell shape and ROCK activity. Decreased cell spreading5,6 and ROCK inhibition7–10 have been shown to promote phenotype retention of chondrocytes. In contrast, expanding chondrocytes in two-dimensional (2D) monolayer culture, where cells spread, induces downregulation of articular cartilage markers and upregulation of undesirable fibroblastic phenotype.11–13
To better maintain chondrocyte phenotype in cartilage tissue engineering, various strategies have been used to reduce cell spreading, such as pellet culture,6 high-density 2D culture,6 suspension culture,14 restricted substrate adhesion culture,5 and three-dimensional hydrogel (3D-HG) culture.7,15,16 In addition to controlling cell shape, other studies also showed that ROCK signaling inhibition using a small-molecule Y27632 led to better chondrocyte phenotype retention.7–10 Together, these studies demonstrate that modulating cell shape and ROCK signaling in chondrocytes can direct impact their ability to produce new cartilage.
While chondrocytes are the native cell type in cartilage and possess strong cartilage producing capacity, their wide clinical application is limited by donor-site morbidity, insufficient supply, and tendency to rapidly dedifferentiate during 2D in vitro expansion.17 To overcome the limitations associated with chondrocytes, mesenchymal stem cells (MSCs) have been extensively explored as an alternative autologous cell source for cartilage regeneration, given their ease of expansion and demonstrated chondrogenic potential.18 Previous studies demonstrate that cell shape regulates osteogenic versus adipogenic differentiation via its effects on ROCK-mediated cytoskeletal tension.4,19
To assess the effects of modulating cell shape on MSC chondrogenesis, various culture models have been used. Reducing MSC spreading by restricting MSC adhesion in 2D,20 seeding in high-density 2D cultures,21 or culturing in pellets20,21 has been shown to increase MSC chondrogenesis. In contrast, the effects of inhibiting ROCK signaling through Y27632 on MSC chondrogenesis are less conclusive and seem to be context dependent. When cultured on 2D tissue culture plastic (TCP) in the presence of Y27632, rat MSCs demonstrated reduced chondrogenic gene expressions.22 However, an opposite trend was reported in a separate study when rat MSCs were cultured on 2D aligned poly(lactic-co-glycolic acid) nanofibers, with Y27632 treatment upregulating chondrogenic gene expressions.23 When human stem cells were encapsulated in 3D collagen hydrogels, Y27632 treatment showed minimal effects on chondrogenic gene expressions.24 For the micromass pellet culture model, most studies show that ROCK inhibition upregulates MSC chondrogenic gene expressions and enhances cartilage matrix deposition.25–27 However, another pellet study reported that Y27632 increased Sox9, but decreased collagen II and aggrecan expression of MSCs.10 Part of the contradictory findings from these previous reports may be due to the wide variety of culture models and biomaterials used in different studies. As such, the effects of Y27632 on MSC chondrogenesis in different culture models remained largely unclear.
In addition to cells, cartilage tissue engineering strategies often involve the use of 3D scaffolds, which provide cells with structural support and protection in a load-bearing environment, and also provide tunable niche cues to promote cartilage deposition. Hydrogels are the most popular scaffolds used for cartilage tissue engineering given their injectability and ability to crosslink in situ to fill defects of any shape. Most conventional hydrogels are nanoporous, requiring degradation before cells can deposit new matrix, which often leads to slow new tissue formation. To overcome this limitation, our laboratory developed gelatin (GEL)-based microribbons (μRBs) that support direct cell encapsulation into a highly macroporous scaffold. Unlike conventional hydrogels, cells encapsulated in the μRB scaffold attach to the surface of individual μRBs and exhibit rapid cell spreading on encapsulation.28 Despite cell spreading, GEL μRB scaffold supported accelerated and enhanced MSC-based new cartilage formation compared with conventional GEL hydrogels, in which cells remain round.29 These results suggest that cell spreading and resulting Rho/ROCK activity did not inhibit chondrogenesis in the μRB culture model like in conventional 2D culture. However, it remained unknown whether ROCK inhibition would further increase the cartilage formation by MSCs in the μRB culture model.
The goal of this study was to investigate the effects of ROCK inhibition on MSC chondrogenesis in the μRB culture model and compare the responses with other culture models, including two-dimensional hydrogel (2D-HG) substrates, conventional 3D-HGs, and pellet culture (Fig. 1A). We chose 2D-HG substrates as a control, given MSCs interact with μRBs like a substrate. For all culture models that involve the use of biomaterials (μRB, 2D-HG, and 3D-HG), we also varied the polymer compositions to assess whether the effects of ROCK inhibition depend on biomaterial choice. Four polymers that have been widely used for cartilage tissue engineering were compared, including GEL,30,31 chondroitin sulfate (CS),32,33 hyaluronic acid (HA),34–37 and polyethylene glycol (PEG).33,36,38 To inhibit ROCK signaling, we used Y27632, a small molecule that has been extensively used in previous studies for evaluating ROCK inhibition on MSC chondrogenesis.10,22–27 All groups were cultured for 21 days in chondrogenic media with or without Y27632. Outcomes were analyzed using live/dead staining, gene expression analyses of mechanosensing and cartilage-specific genes, histology, and immunostaining.
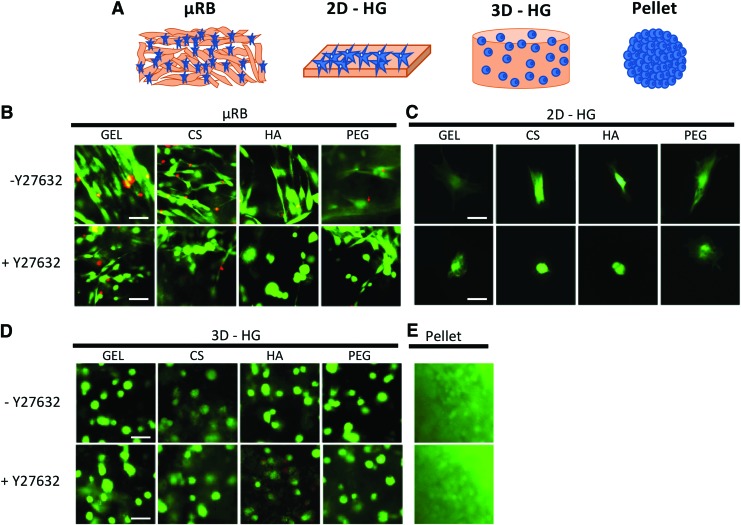
FIG. 1.
(A) Schematic of four culture models used for the comparison, including 3D μRB scaffold, 2D-HG substrates, 3D-HGs, and pellet culture. (B–E) Live/dead staining at 24 h for MSCs in different culture models and biomaterials (gelatin, chondroitin sulfate, hyaluronic acid, and polyethylene glycol). ROCK inhibition was induced using Y27632. Scale bars: 50 μm. μRB, microribbon; MSC, mesenchymal stem cell; 3D-HG, three-dimensional hydrogel; 2D-HG, two-dimensional hydrogel; ROCK, Rho-associated protein kinase. Color images are available online.
Materials and Methods
Synthesis and fabrication of microribbons
GEL μRBs were fabricated using wet-spinning as previously described.28 To fabricate HA and CS μRBs, methacrylated HA (40 kDa; Lifecore) and CS (Sigma-Aldrich) were mixed with 2.5 mM Cys–Arg–Gly–Asp–Ser (CRGDS), loaded into a syringe, and wet-spun into a propanal bath through a G31 needle at 0.5 mL/h. The resulting μRBs were collected and fixed by dithiothreitol. To fabricate PEG μRBs, multiarm PEG maleimide solution (20% [w/v]) was mixed with 2.5 mM CRGDS, injected at 1 mL/h into a 2-propanol bath containing 0.3% (w/v) dithiothreitol, and instantly fixed into the μRB shape. After fixation, all μRBs were washed six times in phosphate-buffered saline (PBS) and lyophilized.
μRB scaffold culture model
To culture MSCs in μRB scaffold, lyophilized μRBs were rehydrated in PBS containing 0.1% lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) for a final μRB density of 7.5% (w/v). Human MSCs (hMSCs) were added to the hydrated μRB precursors for a final cell density of 10 M/mL. The cell-μRB mixtures were then placed between two glass slides (2 mm gap) and photocrosslinked by exposure to UV light (365 nm, 4 mW/cm2, 5 min) to form a cell-laden μRB sheet. After 24 h of incubation in growth media, cylindrical-shaped MSC-laden scaffolds (4 mm in diameter) were punched from the cell-laden μRB sheet using a biopsy punch and transferred to 24-well plates for culture.
Two-dimensional hydrogel substrate culture model
Methacrylated polymers (GEL, CS, HA, and PEG) were dissolved in PBS to achieve final concentrations of 7.5% (w/v). LAP was added to hydrogel precursor solutions at 0.1% (v/v). To form 2D-HG substrates, hydrogel precursors (300 μL) were pipetted into single wells of a 48-well plate and exposed to UV light (365 nm, 4 mW/cm2, 5 min). The resulting hydrogels are 11 mm in diameter and 3 mm in height. A total of 10,000 hMSCs were seeded on top of each 2D-HG.
Three-dimensional hydrogel culture model
Methacrylated molecules (GEL, CS, HA, and PEG) were dissolved in LAP (0.1% in PBS) to achieve a final polymer concentration of 7.5% (w/v). hMSCs were added to the hydrogel precursor solutions for a cell density of 10 M/mL. To form 3D-HGs, a cell/hydrogel mixture (25 μL) was pipetted into a cylindrical mold (4 mm in diameter and 2 mm in height) and exposed to UV light (365 nm, 4 mW/cm2, 5 min) to form a cell-laden hydrogel.
Pellet culture model
To form MSC pellets, 300,000 human MSCs were added to individual wells of a v-bottom 96-well plate and centrifuged at 1000 rpm for 5 min.
Cell culture
Primary human bone marrow-derived MSCs were purchased from a commercial vendor (Lonza) at passage 2. These cells have been well characterized by the vendor to validate MSC markers and differentiation potential. Cells were expanded in growth medium until passage 6 before use. For chondrogenesis culture, all samples were cultured in a standard chondrogenic medium containing 10 ng/mL transforming growth factor-β3, as we previously reported.39 All samples were cultured for 21 days, with medium exchanged every other day. For samples treated with Y27632 (10 μM), 1.5 μL of 10 mM fresh Y26732 solution was added into 1.5 mL chondrogenic medium per well every 24 h. On day 1, cell viability and cell shape were assessed using a LIVE/DEAD Viability/Cytotoxicity Kit (Thermo Fisher).
Gene expression analyses
Gene expression levels of mechanosensing genes (RhoA, ROCK I, and ROCK II) and cartilage-specific genes (Sox9, aggrecan, collagen II, N-cadherin, collagen I, and collagen X) were analyzed on day 7. To extract total RNA, cells on TCP and 2D-HGs were directly lysed, while μRB and 3D-HG samples were first digested in TRIzol (Life Technologies). RNA was precipitated using RNeasy Mini Kit columns (Qiagen). cDNA was synthesized from extracted RNA using the SuperScript III First-Strand Synthesis Kit (Life Technologies). Real time-polymerase chain reaction (RT-PCR) was performed using Power SYBR Green PCR Master Mix (Applied Biosystems, Life Technologies). RT-PCR primers can be found in Supplementary Table S1. Relative expression levels of target genes were determined by the ΔΔCt method. Target gene expression was first normalized to an endogenous housekeeping gene (hGAPDH) followed by a second normalization to the expression level measured in untreated MSCs cultured on TCP at day 1.
Histology and immunostainings
On day 21, scaffolds were fixed in 4% (w/v) paraformaldehyde (Sigma-Aldrich) for 1 h on an orbital shaker. Samples were then embedded in O.C.T. (Tissue-Tek) overnight and frozen in liquid nitrogen the following day. Masson's trichrome staining (Thermo Scientific) and Safranin-O counterstained with fast green were performed to visualize collagen and glycosaminoglycan deposition, respectively. Immunostaining sections were incubated with 0.1% trypsin in PBS for 15 min at 37°C for enzymatic antigen retrieval, 1 h in blocking buffer containing 2% goat serum and 3% bovine serum albumin, and overnight at 4°C in primary antibody solutions. Rabbit Col I, type II collagen (Col II), Col X, lubricin (Abcam), and N-cadherin primary antibodies (Cell Signaling Technology) were diluted 1:100 and added to sample sections separately. The following day, the secondary antibody (Alexa Fluor 488 anti-rabbit) was diluted 1:200 with Hoechst (4 μg/mL) and incubated on sections for 1 h at room temperature.
Statistical analysis
All data are presented as mean ± standard deviation (n ≥ 3/group). GraphPad Prism (GraphPad Software) was used to perform multiple unpaired t-tests for statistical analysis with p < 0.05 indicating statistical significance and p < 0.005 indicating high statistical significance.
Results
ROCK inhibition decreases mesenchymal stem cell spreading in μRBs and on 2D-HG substrates, but does not affect mesenchymal stem cell morphology in 3D-HG or pellet culture
We first assessed the effects of ROCK inhibition on MSC morphology and cell viability in different culture models (Fig. 1A). All biomaterials and culture models supported high cell viability, and Y27632 did not lead to significant changes in cell viability (Fig. 1). When cultured in μRB scaffolds or on 2D-HG substrates, MSCs attached and spread rapidly within 24 h (Fig. 1B, C). Adding Y27632 decreased MSC spreading on both μRB and 2D-HG substrates. In contrast, MSCs were round in 3D-HG and pellet culture and adding Y27632 did not impact cell morphology (Fig. 1D, E).
ROCK inhibition decreases RhoA/ROCK expressions in μRB and 2D culture, but not in 3D-HG or pellet culture
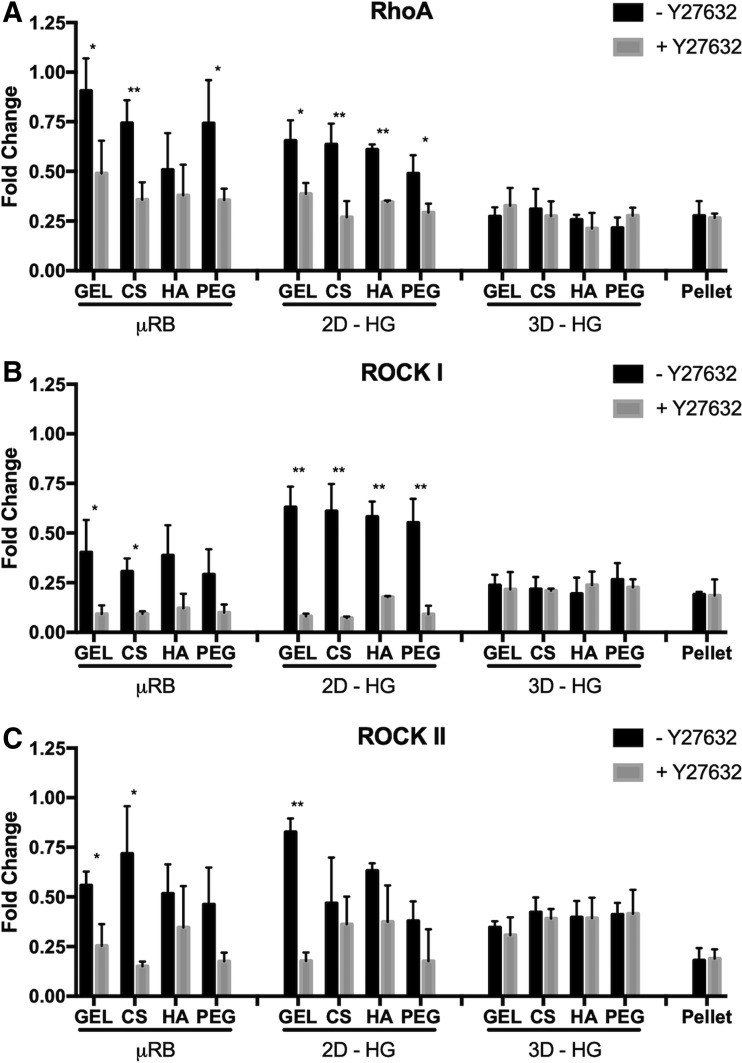
To examine correlation between cell shape and ROCK activity, we performed gene expression analysis of ROCK-related genes. In the absence of Y27632, MSCs cultured in μRB and 2D-HG showed higher expressions of RhoA, ROCKI, and ROCK II compared with 3D-HG and pellet. Y27632 treatment led to downregulation of all three genes in the μRB and 2D-HG culture to a level comparable with those in 3D-HG and pellet. For 3D-HG and pellet culture models, no significant differences were observed in RhoA/ROCK gene expressions with or without Y27632. Furthermore, varying biomaterials compositions had either no or minimal effects on RhoA/ROCK gene expressions across all culture models (Fig. 2).
FIG. 2.
Y27632 significantly decreases expression of (A) RhoA, (B) ROCK I, and (C) ROCK II in μRB and 2D-HG, but has minimal effect in 3D-HG or pellet culture. All gene expressions were reported as fold of change at day 7, normalized to untreated MSCs in monolayer on TCP at day 1. Data are reported as mean ± SD (n = 3/group). *p < 0.05 and **p < 0.005 indicate a statistically significant difference between the paired groups with or without Y27632 within a culture model and the same biomaterial composition. SD, standard deviation; TCP, tissue culture plastic.
ROCK inhibition decreases MSC chondrogenesis in μRB culture and increases MSC chondrogenesis in pellet culture
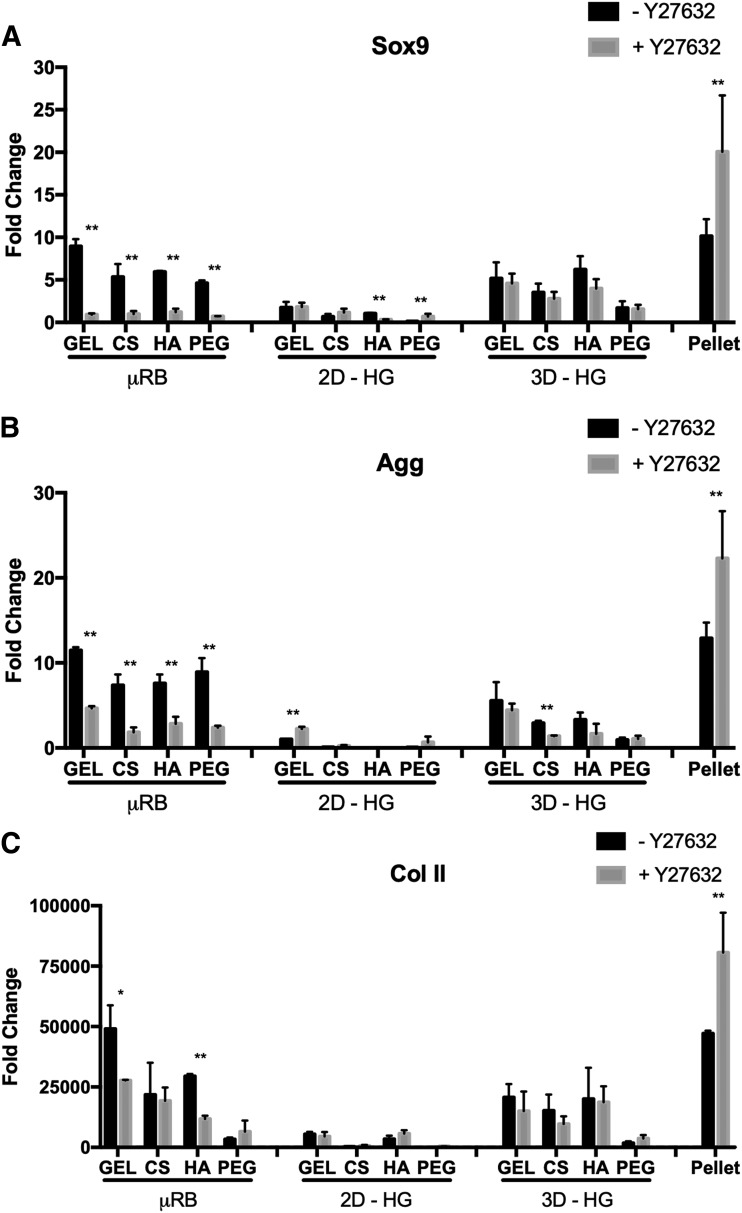
We next assessed the effects of ROCK inhibition on MSC chondrogenesis in different culture models by quantifying gene expression levels of cartilage markers, including Sox9, aggrecan (Agg), and Col II. For the μRB culture model, Y27632 treatment generally led to downregulation of cartilage markers, whereas an opposite trend was observed in pellet culture. Chondrogenic gene expressions were lowest in the 2D-HG culture model and intermediate in the 3D-HG culture model. Unlike the μRB and pellet culture models, 2D-HG and 3D-HG models showed minimal changes in cartilage gene expressions in response to Y27632 (Fig. 3).
FIG. 3.
Y27632 significantly decreases cartilage-specific gene expression in the μRB model, but significantly increases expression in pellet culture, and has minimal effects on 2D-HG or 3D-HG models. Normalized gene expressions of (A) Sox9, (B) aggrecan, and (C) type II collagen. All gene expressions were reported as fold of change at day 7, normalized to untreated MSCs in monolayer on TCP at day 1. Data are reported as mean ± SD (n = 3/group). *p < 0.05 and **p < 0.005 indicate statistically significant difference between the paired groups with or without Y27632 within a culture model and the same biomaterial composition.
While varying biomaterial compositions did not affect the trend of how ROCK inhibition affects MSC chondrogenesis, varying biomaterial compositions did influence the chondrogenic potential of MSCs. Specifically, MSCs in naturally derived biomaterials (GEL, CS, and HA) had higher chondrogenic gene expressions than MSCs in synthetic PEG in both μRB and 3D-HG culture models (Fig. 3).
ROCK inhibition decreases cartilage matrix deposition by MSCs in μRBs, but increases cartilage formation in pellet culture
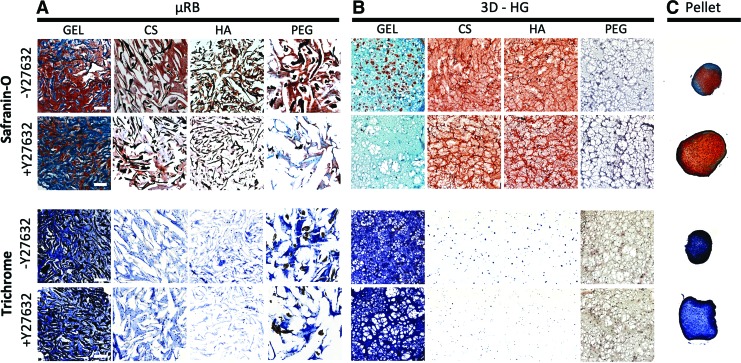
To evaluate the total amount and distribution of newly formed cartilage in different culture models, histology was performed to stain sulfated glycosaminoglycans (sGAG) and total collagen using Safranin-O and Masson's trichrome staining, respectively. Histology was performed for μRB, 3D-HG, and pellet culture models using sectioned specimens. 2D-HGs were excluded from this analysis because they could not be sectioned and took up nonspecific stains as background. For the three culture models examined, we observed the same trend of ROCK inhibition on cartilage matrix deposition in histology (Fig. 4) as previously observed in chondrogenic gene expression analysis (Fig. 3). In brief, Y27632 greatly decreased the amount of sGAG production in μRB culture, minimally affected sGAG production in 3D-HG, and increased sGAG production in pellet culture (Fig. 4). In addition, Y27632 had a minimal effect on total collagen production in μRB and 3D-HG culture models, but increased the total collagen production in pellet culture (Fig. 4).
FIG. 4.
Effects of ROCK inhibition using Y27632 on glycosaminoglycan and collagen deposition in (A) μRB culture, (B) 3D-HG culture, and (C) pellet culture after 21 days in chondrogenic medium. Safranin-O staining was used to visualize glycosaminoglycans, and Masson's trichrome staining was used to visualize total collagen. Scale bars: 250 μm. Color images are available online.
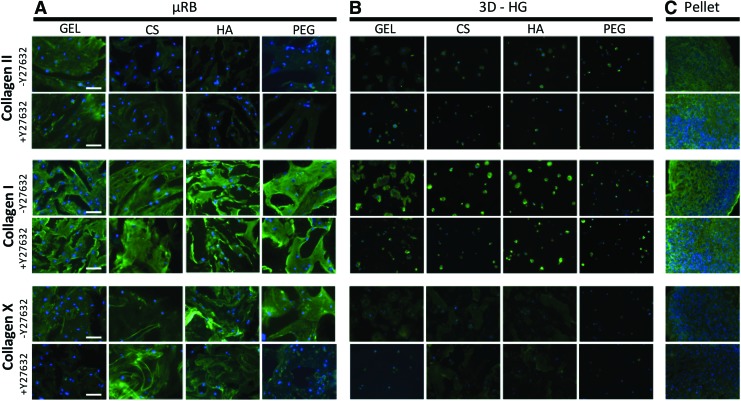
To further assess the phenotype of the resulting cartilage, immunostaining was performed for three markers, including Col II (articular cartilage), type I collagen (fibrocartilage), and type X collagen (hypertrophic cartilage), and quantitative RT-PCR was performed for type I and X collagen. Addition of Y27632 generally did not impact the phenotype of cartilage within specific biomaterials in the μRB culture model (Fig. 5A). Varying polymer compositions, on the contrary, did impact the phenotype of resulting cartilage in the μRB model, with GEL μRBs resulting in the most intense Col II and least type X collagen. In 3D-HG, Y27632 slightly decreased collagen I production, but did not alter collagen II or X production (Fig. 5B). With Y27632, collagen II production increased slightly in pellet culture, while collagen I and X remained constant (Fig. 5C). Both type I and X collagen markers showed only a small increase compared with day 1 (two- to eight-fold) (Supplementary Fig. S1), which is about four orders of magnitude less than the increase in gene expression of Col II, the marker for articular cartilage (Fig. 2). Y27632 had a minimal effect on the gene expression levels of both Col I and Col X markers, with only a few groups showing significant differences (Supplementary Fig. S1).
FIG. 5.
Immunostaining of different types of produced collagen to characterize the phenotype of resulting cartilage in response to ROCK inhibition. Collagen II, I, and X were stained in (A) μRB, (B) 3D-HG, and (C) pellet culture and varying polymer compositions after 21 days in chondrogenic medium. Scale bars: 100 μm. Color images are available online.
Lubricin is a glycoprotein produced by the superficial zone of chondrocytes in articular cartilage. We performed immunostaining of lubricin for three groups, including GEL μRB, GEL 3D-HG, and pellet culture. We chose the GEL groups and pellet as these groups exhibit the most robust cartilage extracellular matrix (ECM) deposition (Fig. 4). Pellet culture showed positive staining for lubricin in both cultures, followed by GEL μRB, and 3D-HG culture showing the least lubricin signals (Supplementary Fig. S2). Y27632 did not cause significant changes in lubricin expression in any group.
In addition, to assess whether changes in cartilage production were due to changes in cell number or changes in the amount of ECM deposition per cell, we stained cell nuclei using 4′,6-diamidino-2-phenylindole staining for the same three groups as above: GEL μRB, GEL 3D-HG, and pellet. While the μRB group and 3D-HG started out with the same cell density, the μRB group showed a much higher cell density than 3D-HG at day 21, indicating that the μRB scaffold supported higher cell proliferation. Y27632 did not lead to noticeable changes in cell density. Pellet showed the highest cell density as expected, and +Y27632 led to a higher cell density by day 21, suggesting that Y27632 increased cell proliferation in pellet culture (Supplementary Fig. S3).
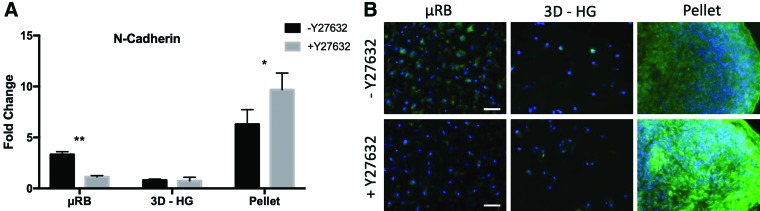
ROCK inhibition significantly decreases N-cadherin in μRB culture and significantly increases N-cadherin in pellet culture
N-cadherin is an important factor in mediating cell/cell interactions during mesenchymal condensation, the required first step of chondrogenesis.40 We speculated that the opposing chondrogenic responses to ROCK inhibition in μRB and pellet culture could have been modulated through N-cadherin. As such, we assessed the effect of Y27632 treatment on N-cadherin expression and production in GEL μRB, GEL 3D-HG, and pellet culture. Because ROCK inhibition decreased chondrogenesis in all μRB biomaterials and minimally affected chondrogenesis in all 3D-HG biomaterials, any biomaterial could have been used to investigate the correlation between ROCK inhibition, N-cadherin, and cartilage deposition. We chose GEL as the biomaterial because it demonstrated the most substantial changes in chondrogenic gene expression in response to Y27632. Quantitative gene expression analyses showed that the expression and production of N-cadherin correlated with cartilage gene expressions and histology. In brief, Y27632 significantly downregulated N-cadherin expression in GEL μRB, but significantly increased N-cadherin expression in pellet culture. No significant change in N-cadherin was observed in the 3D-HG model in response to Y27632 (Fig. 6A). Immunostaining results followed the same trend as gene expressions (Fig. 6B).
FIG. 6.
Characterization of N-cadherin expression using (A) quantitative gene expressions at day 7, normalized to untreated MSCs on TCP at day 1 and (B) immunostaining at day 21. The trend of N-cadherin expression generally followed the same trend as chondrogenesis in response to ROCK inhibition. Data are reported as mean ± SD (n = 3/group). *p < 0.05 and **p < 0.005 indicate statistically significant difference between the paired groups with or without Y27632 within a culture model. Only gelatin was examined here given it was the top performing polymer for both μRB and 3D-HG culture models from previous analyses. Scale bars: 100 μm. Color images are available online.
Discussion
In the present study, we sought to answer whether the effects of ROCK inhibition on MSC chondrogenesis depend on culture models or choice of biomaterials. Our results showed that the effects of ROCK inhibition on MSC chondrogenesis vary depending on culture models. For example, while ROCK inhibition significantly increased MSC chondrogenesis in pellet culture, an opposite trend was observed in μRB culture, and ROCK inhibition had minimal impacts on MSC chondrogenesis in 2D-HG and 3D-HG culture models. Whereas the effect of ROCK inhibition on MSC chondrogenesis varied greatly depending on the culture model, ROCK inhibition led to similar trends in chondrogenesis in all biomaterials tested within a given culture model.
One unexpected finding from this study is that MSC chondrogenesis in the μRB culture is fundamentally different from chondrogenesis on 2D-HG substrates (Fig. 3), despite similar expressions in RhoA/ROCK signaling in the two models (Fig. 2). Unlike electrospun fibers, which are nanoscale, the width of μRBs is on the order of tens to hundreds of microns, larger than the diameter of individual cells. As such, in the μRB culture model, each cell attaches to the surface of only one individual μRB like a substrate. Indeed, MSCs exhibit a comparable level of cell spreading (Fig. 1) and high RhoA/ROCK gene expressions in both μRB culture model and 2D-HG models (Fig. 2). Furthermore, Y27632 treatment led to similar downregulation of RhoA/ROCK signaling in both culture models (Fig. 2). Despite the similarity in ROCK signaling, totally different trends in chondrogenesis were observed in the two culture models. While standard 2D-HG culture inhibits chondrogenesis (Fig. 3), μRB culture supported robust chondrogenesis (Fig. 3) and cartilage formation (Fig. 4). Together, these results suggest MSCs sense μRBs in a much more complex manner than a 2D substrate, and the level of RhoA/ROCK expression cannot be used alone to predict MSC chondrogenesis and cartilage formation.
Unlike the μRB and 2D-HG culture models, MSCs remain round in 3D-HG and pellet culture models due to physical restriction from the nanoporous hydrogel network or cell packing in the pellet. Y27632 treatment did not significantly change cell morphology (Fig. 1D, E) or RhoA/ROCK expressions, which were low in both culture models as expected (Fig. 2). Despite the similarity in RhoA/ROCK gene expressions, Y27632 treatment led to a substantial increase in cartilage formation in the pellet model, but had minimal impacts on the 3D-HG culture model (Fig. 4). These results again validate that MSC chondrogenesis depends on multiple factors, and ROCK signaling alone cannot be used to predict MSC-based cartilage formation.
While the effects of ROCK inhibition on MSC chondrogenesis are opposite in μRB and pellet culture, we observed a consistent trend in the correlation between increased cartilage gene expressions (Fig. 3) and increased N-cadherin expressions (Fig. 6) in both culture models. In the μRB model, N-cadherin expression was higher when cells were spread (−Y27632), as they were probably more likely to form cell/cell contacts. Y27632 treatment reduced cell spreading in the μRB model (Fig. 1B), decreasing cell/cell contact and downregulating N-cadherin expression (Fig. 6A). In the pellet culture, however, cell/cell contacts were present with or without Y27632. Similar to our finding, previous studies using the pellet culture model also reported that ROCK inhibition enhanced chondrogenic gene expressions and cartilage matrix deposition by MSCs.25–27 Y27632 treatment may decrease cytoskeletal tension,41 contact modulus,42 or cortical stiffness.43 Our finding that increased N-cadherin is correlated with enhanced chondrogenesis is consistent with previous reports. For example, HA hydrogels functionalized with N-cadherin mimetic peptides, or self-assembling N-cadherin mimetic peptide hydrogels have been shown to accelerate chondrogenesis and cartilage matrix formation.37,44
In summary, here we demonstrate that the effects of ROCK inhibition on MSC chondrogenesis are highly dependent on the culture model. Importantly, while different culture models may exhibit similar RhoA/ROCK levels, such as μRB and 2D-HGs, or pellet and 3D-HGs, their chondrogenesis responses can be totally different. These results suggest that MSC chondrogenesis depends on multiple factors and cannot be predicted using ROCK signaling alone. While varying biomaterial compositions may impact the amount of cartilage formation and phenotype of resulting cartilage, varying biomaterials did not change the chondrogenic response to ROCK inhibition within each culture model. Regardless of culture models or ROCK expressions, increased cartilage formation was always accompanied by enhanced N-cadherin expression and production, suggesting that N-cadherin is a robust marker to select culture models and conditions that promote chondrogenesis. Together, the results from this study fill in a gap of knowledge in the correlation between ROCK signaling and MSC chondrogenesis, which may be used to enhance MSC-based cartilage regeneration in different culture models.
Supplementary Material
Acknowledgment
C.A.G. thanks the Stanford Bio-X Graduate Fellowship for support.
Disclosure Statement
No competing financial interests exist.
Funding Information
The authors thank NIH R01DE024772, California Institute for Regenerative Medicine Tools and Technologies Award (Grant #RT3-07804), National Science Foundation CAREER award program (CBET-1351289), the Stanford Bio-X Interdisciplinary Initiative Program (F.Y.), and the Stanford Child Health Research Institute Faculty Scholar Award (F.Y.). C.G. would like to thank Stanford Bio-X Graduate Fellowship for support.
Supplementary Material
References
- 1. Thorogood P.V., and Hinchliffe J.R.. An analysis of the condensation process during chondrogenesis in the embryonic chick hind limb. J Embryol Exp Morphol 33, 581, 1975 [PubMed] [Google Scholar]
- 2. Kosher R.A., Kulyk W.M., and Gay S.W.. Collagen gene expression during limb cartilage differentiation. J Cell Biol 102, 1151, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bhadriraju K., Yang M., Alom Ruiz S., Pirone D., Tan J., and Chen C.S.. Activation of ROCK by RhoA is regulated by cell adhesion, shape, and cytoskeletal tension. Exp Cell Res 313, 3616, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McBeath R., Pirone D.M., Nelson C.M., Bhadriraju K., and Chen C.S.. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell 6, 483, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Glowacki J., Trepman E., and Folkman J.. Cell shape and phenotypic expression in chondrocytes. Proc Soc Exp Biol Med 172, 93, 1983 [DOI] [PubMed] [Google Scholar]
- 6. Abbott J., and Holtzer H.. The loss of phenotypic traits by differentiated cells. 3. The reversible behavior of chondrocytes in primary cultures. J Cell Biol 28, 473, 1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tew S.R., and Hardingham T.E.. Regulation of SOX9 mRNA in human articular chondrocytes involving p38 MAPK activation and mRNA stabilization. J Biol Chem 281, 39471, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Matsumoto E., Furumatsu T., Kanazawa T., Tamura M., and Ozaki T.. ROCK inhibitor prevents the dedifferentiation of human articular chondrocytes. Biochem Biophys Res Commun 420, 124, 2012 [DOI] [PubMed] [Google Scholar]
- 9. Furumatsu T., Matsumoto-Ogawa E., Tanaka T., Lu Z., and Ozaki T.. ROCK inhibition enhances aggrecan deposition and suppresses matrix metalloproteinase-3 production in human articular chondrocytes. Connect Tissue Res 55, 89, 2014 [DOI] [PubMed] [Google Scholar]
- 10. Woods A., and Beier F.. RhoA/ROCK signaling regulates chondrogenesis in a context-dependent manner. J Biol Chem 281, 13134, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Mayne R., Vail M.S., Mayne P.M., and Miller E.J.. Changes in type of collagen synthesized as clones of chick chondrocytes grow and eventually lose division capacity. Proc Natl Acad Sci U S A 73, 1674, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. von der Mark K., Gauss V., von der Mark H., and Muller P.. Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature 267, 531, 1977 [DOI] [PubMed] [Google Scholar]
- 13. Ma B., Leijten J.C., Wu L., et al. . Gene expression profiling of dedifferentiated human articular chondrocytes in monolayer culture. Osteoarthritis Cartilage 21, 599, 2013 [DOI] [PubMed] [Google Scholar]
- 14. Kolettas E., Buluwela L., Bayliss M.T., and Muir H.I.. Expression of cartilage-specific molecules is retained on long-term culture of human articular chondrocytes. J Cell Sci 108 (Pt 5), 1991, 1995 [DOI] [PubMed] [Google Scholar]
- 15. Benya P.D., and Shaffer J.D.. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell 30, 215, 1982 [DOI] [PubMed] [Google Scholar]
- 16. Aulthouse A.L., Beck M., Griffey E., et al. . Expression of the human chondrocyte phenotype in vitro. In Vitro Cell Dev Biol 25, 659, 1989 [DOI] [PubMed] [Google Scholar]
- 17. Grande D.A., Breitbart A.S., Mason J., Paulino C., Laser J., and Schwartz R.E.. Cartilage tissue engineering: current limitations and solutions. Clin Orthopaed Relat Res S176, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Solchaga L.A., Penick K.J., and Welter J.F.. Chondrogenic differentiation of bone marrow-derived mesenchymal stem cells: tips and tricks. Methods Mol Biol 698, 253, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kilian K.A., Bugarija B., Lahn B.T., and Mrksich M.. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc Natl Acad Sci U S A 107, 4872, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gao L., McBeath R., and Chen C.S.. Stem cell shape regulates a chondrogenic versus myogenic fate through Rac1 and N-cadherin. Stem Cells 28, 564, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eyckmans J., Lin G.L., and Chen C.S.. Adhesive and mechanical regulation of mesenchymal stem cell differentiation in human bone marrow and periosteum-derived progenitor cells. Biol Open 1, 1058, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xu T., Wu M., Feng J., Lin X., and Gu Z.. RhoA/Rho kinase signaling regulates transforming growth factor-beta1-induced chondrogenesis and actin organization of synovium-derived mesenchymal stem cells through interaction with the Smad pathway. Int J Mol Med 30, 1119, 2012 [DOI] [PubMed] [Google Scholar]
- 23. Zhong W., Zhang W., Wang S., and Qin J.. Regulation of fibrochondrogenesis of mesenchymal stem cells in an integrated microfluidic platform embedded with biomimetic nanofibrous scaffolds. PLoS One 8, e61283, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. ZuFu L., Behrouz Zandieh D., ChunLing H., Ruud A.B., and Marco N.H.. Collagen type II enhances chondrogenesis in adipose tissue–derived stem cells by affecting cell shape. Tissue Eng Part A 16, 81, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Woods A., Wang G., and Beier F.. RhoA/ROCK signaling regulates Sox9 expression and actin organization during chondrogenesis. J Biol Chem 280, 11626, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Wang K.C., Egelhoff T.T., Caplan A.I., Welter J.F., and Baskaran H.. ROCK inhibition promotes the development of chondrogenic tissue by improved mass transport. Tissue Eng Part A 24, 1218, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhao Y.H., Lv X., Liu Y.L., et al. . Hydrostatic pressure promotes the proliferation and osteogenic/chondrogenic differentiation of mesenchymal stem cells: the roles of RhoA and Rac1. Stem Cell Res 14, 283, 2015 [DOI] [PubMed] [Google Scholar]
- 28. Han L.-H., Yu S., Wang T., Behn A.W., and Yang F.. Microribbon-like elastomers for fabricating macroporous and highly flexible scaffolds that support cell proliferation in 3D. Adv Funct Mater 23, 346, 2013 [Google Scholar]
- 29. Conrad B., Han L.H., and Yang F.. Gelatin-based microribbon hydrogels accelerate cartilage formation by mesenchymal stem cells in 3D. Tissue Eng Part A 24, 1631, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ponticiello M.S., Schinagl R.M., Kadiyala S., and Barry F.P.. Gelatin-based resorbable sponge as a carrier matrix for human mesenchymal stem cells in cartilage regeneration therapy. J Biomed Mater Res 52, 246, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Schuurman W., Levett P.A., Pot M.W., et al. . Gelatin-methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs. Macromol Biosci 13, 551, 2013 [DOI] [PubMed] [Google Scholar]
- 32. Wang T., and Yang F.. A comparative study of chondroitin sulfate and heparan sulfate for directing three-dimensional chondrogenesis of mesenchymal stem cells. Stem Cell Res Therapy 8, 284, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Varghese S., Hwang N.S., Canver A.C., Theprungsirikul P., Lin D.W., and Elisseeff J.. Chondroitin sulfate based niches for chondrogenic differentiation of mesenchymal stem cells. Matrix Biol 27, 12, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Bian L., Hou C., Tous E., Rai R., Mauck R.L., and Burdick J.A.. The influence of hyaluronic acid hydrogel crosslinking density and macromolecular diffusivity on human MSC chondrogenesis and hypertrophy. Biomaterials 34, 413, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhu M., Feng Q., and Bian L.. Differential effect of hypoxia on human mesenchymal stem cell chondrogenesis and hypertrophy in hyaluronic acid hydrogels. Acta Biomater 10, 1333, 2014 [DOI] [PubMed] [Google Scholar]
- 36. Chung C., and Burdick J.A.. Influence of three-dimensional hyaluronic acid microenvironments on mesenchymal stem cell chondrogenesis. Tissue Eng Part A 15, 243, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bian L., Guvendiren M., Mauck R.L., and Burdick J.A.. Hydrogels that mimic developmentally relevant matrix and N-cadherin interactions enhance MSC chondrogenesis. Proc Natl Acad Sci U S A 110, 10117, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Williams C.G., Kim T.K., Taboas A., Malik A., Manson P., and Elisseeff J.. In vitro chondrogenesis of bone marrow-derived mesenchymal stem cells in a photopolymerizing hydrogel. Tissue Eng 9, 679, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Wang T., Lai J.H., and Yang F.. Effects of hydrogel stiffness and extracellular compositions on modulating cartilage regeneration by mixed populations of stem cells and chondrocytes in vivo. Tissue Eng Part A 22, 1348, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Oberlender S.A., and Tuan R.S.. Expression and functional involvement of N-cadherin in embryonic limb chondrogenesis. Development 120, 177, 1994 [DOI] [PubMed] [Google Scholar]
- 41. Tinevez J.Y., Schulze U., Salbreux G., Roensch J., Joanny J.F., and Paluch E.. Role of cortical tension in bleb growth. Proc Natl Acad Sci U S A 106, 18581, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nijenhuis N., Zhao X., Carisey A., Ballestrem C., and Derby B.. Combining AFM and acoustic probes to reveal changes in the elastic stiffness tensor of living cells. Biophys J 107, 1502, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Srinivasan S., Ashok V., Mohanty S., et al. . Blockade of Rho-associated protein kinase (ROCK) inhibits the contractility and invasion potential of cancer stem like cells. Oncotarget 8, 21418, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li R., Xu J., Wong D.S.H., Li J., Zhao P., and Bian L.. Self-assembled N-cadherin mimetic peptide hydrogels promote the chondrogenesis of mesenchymal stem cells through inhibition of canonical Wnt/beta-catenin signaling. Biomaterials 145, 33, 2017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.