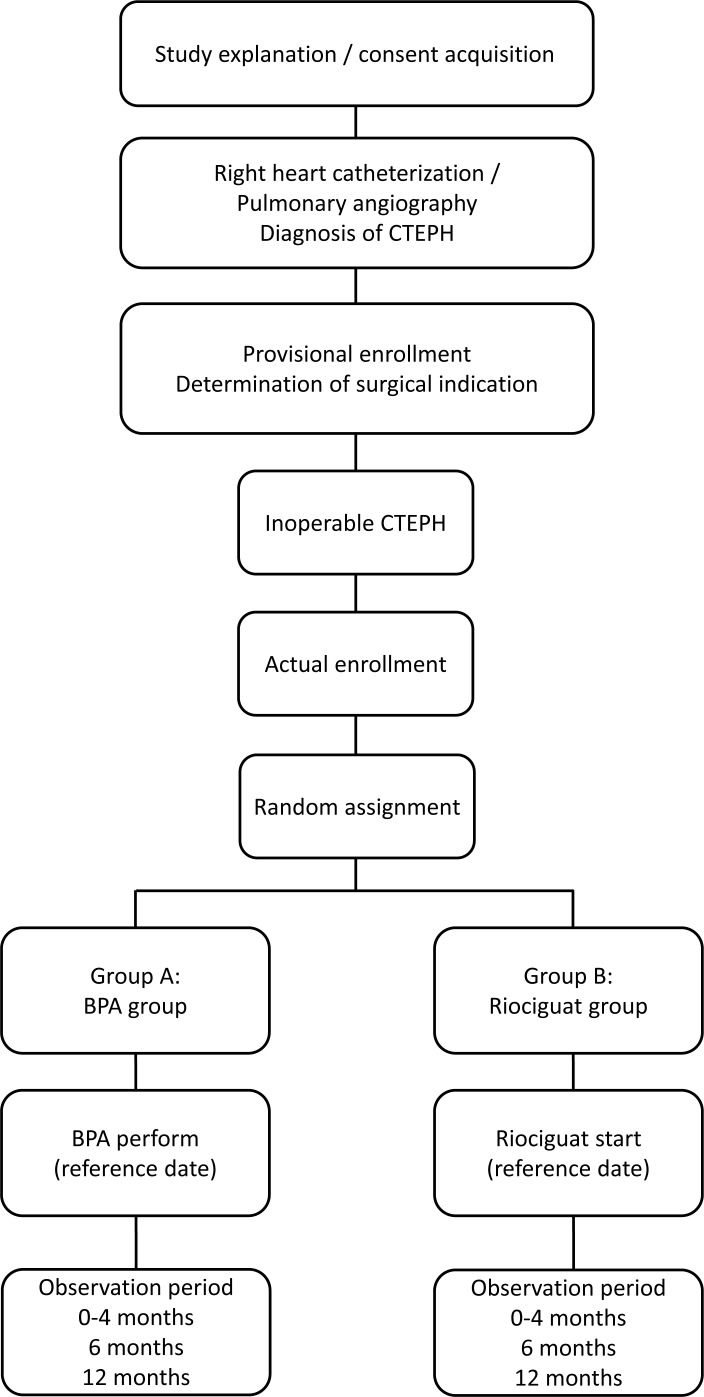
Figure 1.
Flow diagram of study recruitment and randomisation. After obtaining consent and diagnosis of chronic thromboembolic pulmonary hypertension is confirmed, subjects will be randomised into either the balloon pulmonary angioplasty or riociguat group, to receive treatment for 12 months. Observations will be recorded at the time of screening; at baseline and at 0–4, 6 and 12 months after the initiation of treatment. BPA, balloon pulmonary angioplasty; CTEPH, chronic thromboembolic pulmonary hypertension.