Abstract
Inflammation plays a crucial part in hyperglycemia-induced myocardial damage. Hydrogen sulfide has been found to possess multiple biological activities in previous studies. This study investigated whether hydrogen sulfide conferred cardiac protection against damage in a diabetic rat model by inhibiting nucleotide-binding oligomerization domain-like receptor protein (NLRP) 3 inflammasome activation. Male animals were assigned to control, streptozotocin, streptozotocin + sodium hydrosulfide, and streptozotocin + DL-propargylglycine groups. Animals in the three streptozotocin groups were administrated 55 mg/kg streptozotocin by intraperitoneal injection. Streptozotocin + sodium hydrosulfide and streptozotocin + propargylglycine groups were treated with sodium hydrosulfide (56 μmol/kg) and propargylglycine (40 mg/kg), respectively, for four weeks. Estimation of fasting blood glucose, heart-weight/body-weight, cardiac function, and histopathological analysis, and measurement of myocardial enzymes were done to evaluate the degree of cardiac injury. In order to investigate the redox changes, the levels of total antioxidant capacity, malondialdehyde and lipid peroxidation, and the activities of superoxide dismutase, catalase, and glutathione peroxidase were assessed; the protein expression levels of Thioredoxin and Thioredoxin-interacting protein were measured in myocardial tissue. In addition, inflammatory reactions were assessed by measuring the concentration levels of interleukin-6, tumor necrosis factor-α, interleukin-1β, and interleukin-18 in serum and the expression levels of NLRP3 inflammasome complex-associated proteins in cardiac tissue. In the heart, hyperglycemia significantly induced cardiac dysfunction and injury, redox perturbation, and aggravation of inflammatory reactions. However, except for fasting blood glucose, treatment with sodium hydrosulfide significantly ameliorated these alterations, whereas treatment with propargylglycine further aggravated these alterations. This study highlights the protective properties of hydrogen sulfide against hyperglycemia-induced cardiac injury, and its possible mechanism was shown to involve negative regulation of Thioredoxin-interacting protein-mediated NLRP3 inflammasome activation.
Impact statement
Diabetic cardiomyopathy is a serious complication of diabetic patients, accompanied by chronic inflammation. The nucleotide-binding oligomerization domain-like receptor protein (NLRP) 3 inflammasome complex is involved in the progression of the inflammatory response of diabetes, including diabetic cardiomyopathy. Hydrogen sulfide (H2S) is a novel endogenous gas messenger. Several pieces of evidence have exhibited that H2S exerts anti-oxidant and anti-inflammatory activities against hyperglycemia-induced myocardial injury, but the mechanism remains unclear. The current study indicated that H2S protected the myocardium against hyperglycemia-induced injury by preventing Thioredoxin-interacting protein (TXNIP)-mediated NLRP3 inflammasome complex activation. The inhibition of TXNIP-mediated NLRP3 inflammasome complex would be an efficient therapy for H2S treatment in diabetic cardiomyocytes.
Keywords: Hydrogen sulfide, diabetes mellitus, rat, inflammation, oxidative stress, nucleotide-binding oligomerization domain-like receptor protein 3 inflammasome
Introduction
Diabetes mellitus is a group of chronic metabolic diseases that causes high blood glucose concentrations and a number of serious complications, such as nervous, renal, retinal, and cardiovascular diseases.1 Diabetic cardiomyopathy (DCM) is a heart disease caused by diabetes mellitus.2 DCM, characterized by a decreased diastolic and systolic cardiac function and not associated with hypertension and coronary heart disease, is the salient cause of death in patients with diabetes.3 One of the main causes of DCM is low-grade chronic inflammation in myocardial tissue.4
The nucleotide-binding oligomerization domain-like receptor protein (NLRP) 3 inflammasome is a well-known inflammasome complex, which is considered as an important regulator of inflammatory reaction in diabetes mellitus.5 The NLRP3 inflammasome is mainly comprised of NLRP3 protein, apoptosis-associated speck-like protein (ASC), and procaspase-1.6 Stimulation of the NLRP3 inflammasome sparks activation of procaspase-1 and subsequent cleavage of pro-interleukin (IL)-18 and pro-IL-1β results in their activation to pro-inflammatory cytokines.7 TXNIP is a negative regulatory factor of Thioredoxin, which combines with Thioredoxin and inhibits the redox-regulatory activity of Thioredoxin.8 The expression level of TXNIP protein is powerfully stimulated by extracellular high glucose level, which is now considered as a crucial activator of NLRP3 inflammasome in vivo.9 Many reports have stated that inhibition of NLRP3 inflammasome significantly alleviates DCM.10,11
H2S is a novel gas signaling molecule in mammals and can be produced from L-cysteine by several endogenous enzymes, including cystathionine-γ-lyase (CSE), 3-mercaptopyruvate sulfurtransferase, and cystathionine-β-synthase.12 Sodium hydrosulfide (NaHS) often serves as an exogenous donor of H2S, while DL-propargylglycine (PAG) is an irreversible inhibitor of CSE, which is responsible for H2S generation in the cardiovascular system.13 In recent years, evidence shows that H2S has an increasingly salient contribution in the physiological and pathological processes of the heart.14 Recent evidence suggests that H2S has protective properties against inflammation and cell death in the diabetic heart, although it does not reduce high blood glucose level.15 Exogenous H2S is also reported to protect H9c2 cardiomyocytes from high glucose-induced damage by suppressing NLRP3 inflammasome activation.16 However, in type 1 diabetic rats, whether H2S protects the myocardium against injury by inhibiting NLRP3 inflammasome activation remains unclear. Hence, in this work, we explored the therapeutic effect of H2S on cardiac inflammation in diabetic rats and elucidated its mechanism, i.e. whether it is related to inhibition of TXNIP-mediated NLRP3 inflammasome activation.
Materials and methods
Animals
Twenty-eight healthy male Sprague-Dawley rats (weighing 180–220 g) aged 7–8 weeks were acquired from the Experimental Animal Center of Bengbu Medical College, Bengbu, China. All the animals were kept at a room temperature-controlled environment at 23 ± 1°C under a 12 h light–12 h dark cycle and were offered a standard rodent diet and water ad libitum. All experimental procedures were approved by the Animal Experimentation Ethics Committee of Bengbu Medical College.
Chemicals and reagents
NaHS, PAG, and streptozotocin (STZ) were provided by Sigma-Aldrich, USA. Creatine kinase (CK), glutathione peroxidase (GSH-Px), catalase, malondialdehyde (MDA), total antioxidant capacity (T-AOC), lactate dehydrogenase (LDH), superoxide dismutase (SOD), and lipid peroxidation (LPO) assay kits were acquired from Jiancheng Bioengineering Institute, Nanjing, China. ELISA kits for detection of IL-6, IL-18, tumor necrosis factor (TNF)-α, and IL-1β were provided by Neobioscience, Shenzhen, China. Primary antibodies against Thioredoxin and TXNIP were provided by Abcam, UK. Primary antibody against ASC was provided by Santa Cruz Biotechnology, USA. Primary antibodies against NLPR3, caspase-1 (p20), and β-actin and secondary antibodies were provided by Boster Biotechnology, Wuhan, China.
Experimental design
Animals were arranged randomly into four groups of seven rats each: control (CON), STZ, STZ + NaHS (SH), and STZ + PAG (SP). A rat model of type 1 diabetes was established in accordance with our previously reported method.13 Briefly, the overnight-fasted animals in the three STZ groups were injected intraperitoneally (i.p.) with 55 mg/kg STZ. Then, 72 h after STZ injection, the overnight-fasted animals with hyperglycemia (blood glucose over 16.7 mM) were considered diabetic. From the fifth week after successful establishment of the diabetes model, the rats assigned to the SH and SP groups were administrated NaHS (56 μmol/kg/day) and PAG (40 mg/kg/day), i.p., respectively, for 28 days. Animals assigned to the other two groups were administrated with the same volume of 0.9% NaCl solution i.p. for the same duration. The level of fasting blood glucose (FBG) was monitored weekly during the experimental period.
Cardiac function measurement
After different treatments, rats were weighed and anesthetized with an injection i.p. of 350 mg/kg chloral hydrate. Cardiac function-related parameters, including left ventricular (LV) end-diastolic pressure (LVEDP), LV systolic pressure (LVSP), as well as maximal rise/fall rate of LV pressure (±dp/dtmax), were measured using the method we previously described.13
Determination of FBG and heart-weight/body-weight
After cardiac function measurement, the blood sample from each rat was gathered and the FBG level was measured. After rats were sacrificed, their hearts were removed and weighed. Then the heart-weight (HW)/body-weight (BW) ratio was calculated.
Determination of activities of cardiac enzyme
The centrifuge tubes containing blood were centrifuged and the sera were subsequently harvested to examine CK and LDH activities using protocols supplied by the manufacturer.
Determination of levels of serum inflammatory cytokines
Serum inflammatory cytokines including IL-6, TNF-α, IL-1β, and IL-18 were detected using ELISA kits in accordance with the protocols of corresponding assay kits.
Histopathological and ultrastructural evaluation
For histopathological analysis, fresh cardiac tissues were harvested, soaked in 4% paraformaldehyde solution, and subsequently fixed in paraffin wax. Heart slices (5 μm) were dyed with hematoxylin and eosin (H&E) staining kit (Beyotime Biotechnology, China) and Masson’s trichrome reagents, respectively. Sections were examined with a NanoZoomer 2.0 RS digital pathology slide scanner (magnification, ×400).
For the ultrastructural study, fresh heart tissue was cut into the size of 1 mm × 1 mm × 1 mm, and the ultrathin slices were made and stained in accordance with the reported method.13 These ultrathin slices were analyzed under a JEM-1230 transmission electron microscope.
Estimation of biochemical indicators
Heart tissues (100 mg) were homogenized in a nine-fold volume of freezing 0.9% NaCl solution. After centrifugation, the supernatant fluids were obtained. After determining the protein concentrations, the myocardial T-AOC, catalase, SOD, GSH-Px, MDA, and LPO levels were examined using the corresponding assay kits.
Immunohistochemical analysis
Five micrometer-thickness myocardial slices embedded in paraffin wax were dewaxed and then rehydrated. After heating for antigen retrieval, the slices were covered with 3% hydrogen peroxide to prevent the activity of myocardial peroxidase. The sections were treated with 3% bovine serum albumin and subsequently treated with anti-Thioredoxin and anti-TXNIP rabbit polyclonal antibodies, respectively. All the myocardial slices were then incubated with secondary immunoglobulin G (IgG) antibody. After washing with PBS, sections were treated using diaminobenzidine and then dyed with hematoxylin, dehydrated, and mounted. Immunohistochemical results were observed and imaged by the digital pathology slide scanner (magnification, ×400). Four views of each slice were chosen randomly and calculated via Image Pro-Plus 6.0 software.
Western blot analysis
Cardiac tissue lysate was extracted using a lysis buffer with phenylmethanesulfonyl fluoride, and the myocardial protein content was quantified by a bicinchoninic acid kit. A total of 40 μg of proteins were isolated using SDS-PAGE and subsequently electroblotted onto polyvinylidene difluoride membranes. After immersing with 5% nonfat milk powder in Tris-buffered saline Tween-20 for 2 h, all the membranes were probed with primary antibodies against NLRP3, β-actin, ASC, and caspase-1 (p20) at 4°C, overnight. Subsequently, membranes were immersed in proper horseradish peroxidase-linked secondary IgG antibodies. Finally, the immunoreactive band was visualized after incubation with an enhanced chemiluminescence solution and then imaged using a ChemiDoc XRS system. The relative band density was normalized to that of the β-actin loading CON and analyzed using Quantity One software.
Statistical analysis
Statistical analyses were done with SPSS 17.0 software. Experimental results were shown as means ± SD. Data were analyzed using one-way analysis of variance followed by the Newman-Keuls test. Statistical significance was set at P-value less than 0.05.
Results
Effect of H2S on FBG and HW/BW
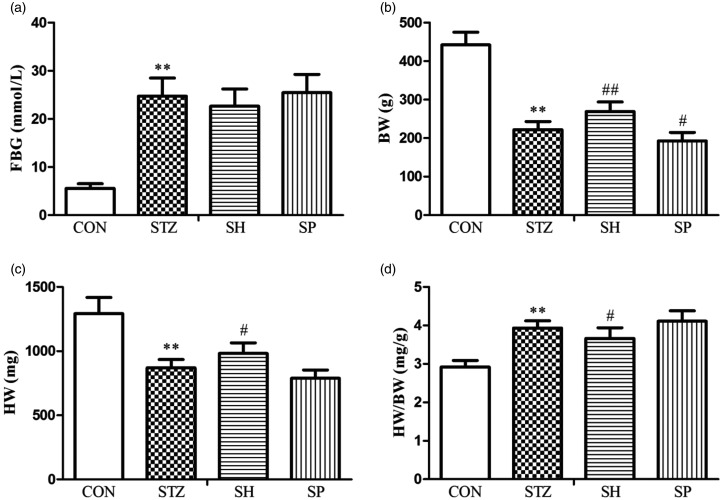
As shown in Figure 1, FBG and HW/BW were increased remarkably, whereas BW and HW were decreased in the STZ group compared with the CON group, which indicated that diabetes causes cardiac hypertrophy. Compared with the STZ group, except for FBG, NaHS treatment reduced the ratio of HW/BW, and increased BW and HW in the SH group. On the other hand, there were no statistically significant differences in FBG, HW, and HW/BW, whereas BW was decreased in the SP group in contrast to the STZ group. The findings showed that H2S ameliorated cardiac hypertrophy in diabetic rats.
Figure 1.
Effects of H2S on FBG and HW/BW in rats. (a) FBG. (b) BW. (c) HW. (d) HW/BW. Values, mean ± SD; n = 7; **P < 0.01 vs. CON group; #P < 0.05, ##P < 0.01 vs. STZ group; CON: control group; STZ: streptozotocin group; SH: STZ + sodium hydrosulfide group; SP: STZ + DL-propargylglycine group.
Effect of H2S on cardiac function
Significant impairment of cardiac function was observed in the STZ group, including a rise in LVEDP and a decline in LVSP and ±dp/dtmax. NaHS treatment enhanced cardiac systolic and diastolic function remarkably in contrast to the STZ group. On the other hand, PAG treatment aggravated cardiac systolic and diastolic dysfunction in contrast to the STZ group (Figure 2).
Figure 2.
Effects of H2S on cardiac functional parameters in rats. (a) LVEDP. (b) LVSP. (c) +dp/dtmax. (d) –dp/dtmax. Values, mean ± SD; n = 7; **P < 0.01 vs. CON group; #P < 0.05, ##P < 0.01 vs. STZ group; CON: control group; STZ: streptozotocin group; SH: STZ+sodium hydrosulfide group; SP: STZ+DL-propargylglycine group.
Effects of H2S on cardiac enzymes and inflammatory cytokines in serum
As shown in Figure 3, STZ injection to rats resulted in cardiac damage and inflammatory reaction; the levels of serum cardiac enzymes (CK and LDH) and pro-inflammatory factors (IL-6, TNF-α, IL-18, and IL-1β) were raised remarkably in the STZ group in contrast to the CON group. On treatment with exogenous H2S, the levels of cardiac enzymes and pro-inflammatory factors in serum were significantly reduced in the SH group in contrast to the STZ group. On the other hand, on treatment with PAG to inhibit the production of endogenous H2S, the same indicators in serum were further raised in the SP group in contrast to the STZ group, suggesting that H2S could ameliorate STZ-induced cardiac damage and inflammatory reaction.
Figure 3.
Effects of H2S on cardiac enzymes and inflammatory cytokines in serum. (a) LDH. (b) CK. (c) IL-6. (d) TNF-α. (e) IL-1β. (f) IL-18. Values, mean ± SD; n = 7; **P < 0.01 vs. CON group; #P < 0.05, ##P < 0.01 vs. STZ group; CON: control group; STZ: streptozotocin group; SH: STZ+sodium hydrosulfide group; SP: STZ+DL-propargylglycine group.
Effect of H2S on histopathological and ultrastructural alterations
H&E staining revealed that the myofibers were arranged neatly and orderly, with clear cell nuclei and minimal inflammatory cell infiltration in the normal heart tissue. The myofibers were disordered with inflammatory cell infiltration in cardiac tissue in the STZ group. Compared with the STZ group, on treatment with NaHS, myocardial injury was evidently alleviated, and most of the myofibers were arranged in neat rows, and infiltration of inflammatory cells in the SH group was further reduced; after treatment with PAG, the myocardial injury deteriorated further in the SP group (Figure 4(a)).
Figure 4.
Effects of H2S on H&E staining (×400), Masson’s trichrome staining (×400), and ultrastructural alterations (×8000) in myocardial tissues. CON: control group; STZ: streptozotocin group; SH: STZ+sodium hydrosulfide group; SP: STZ+DL-propargylglycine group. (A color version of this figure is available in the online journal.)
Masson’s trichrome staining showed normal cardiac myocytes with less collagen deposition in the CON group. A lot of blue-stained collagen fibers were deposited in the diabetic hearts in the STZ group. On treatment with exogenous H2S, the deposition of collagen fibers evidently declined in the SH group, whereas the deposition of collagen fibers was further increased in the SP group in contrast to the STZ group (Figure 4(b)).
Ultrastructural examination of the normal hearts showed normal architecture of cardiac myocytes. The integrity of the cardiomyocytes in diabetic sections was destroyed, the myofibers were partially broken and even dissolved, and the mitochondria were swollen. In the SH group, the ultrastructural injury was evidently ameliorated, whereas the severity of cardiomyocyte injury was further aggravated in the SP group (Figure 4(c)).
Effect of H2S on redox status in DCM
STZ injection to rats resulted in hyperglycemia-triggered oxidative stress and disturbed the balance of redox state; the results showed that the level of T-AOC and the activities of catalase, GSH-Px, and SOD were decreased, whereas the levels of MDA and LPO were raised in myocardial tissues from diabetic rats in contrast to the CON group. Treatment with NaHS downregulated the oxidative status of myocardial tissue in the SH group in contrast to the diabetic rats. On the other hand, after treatment with PAG, the above-mentioned indicators further deteriorated in the SP group compared with the diabetic rats (Figure 5).
Figure 5.
Effects of H2S on redox status in myocardial tissue in rats. (a) T-AOC. (b) SOD. (c) GSH-Px. (d) catalase. (e) MDA. (f) LPO. Values, mean ± SD; n = 7; **P < 0.01 vs. CON group; #P < 0.05, ##P < 0.01 vs. STZ group; CON: control group; STZ: streptozotocin group; SH: STZ + sodium hydrosulfide group; SP: STZ + DL- propargylglycine group.
Immunohistochemical results exhibited that compared with the CON group, the expression level of Thioredoxin protein was reduced, whereas the expression level of TXNIP protein was raised in myocardial tissue from the STZ group. On treatment with NaHS, the expression level of Thioredoxin protein was elevated, whereas the expression level of TXNIP protein was reduced remarkably in the SH group in contrast to the diabetic rats. However, treatment with PAG significantly promoted TXNIP expression further and reduced Thioredoxin expression in the SP group in contrast to diabetic rats (Figure 6). These findings showed that H2S improved the balance of the redox status in diabetic cardiomyocytes.
Figure 6.
Effects of H2S on the protein expressions of Thioredoxin and TXNIP in myocardial tissues. (a) Immunohistochemical studies of Thioredoxin and TXNIP were accessed in rat myocardial tissues of each group (×400). (b) The ratio of Thioredoxin-positive cells in rat myocardial tissues of each group. (c) The ratio of TXNIP-positive cells in rat myocardial tissues of each group. Values, mean ± SD; n = 7; **P < 0.01 vs. CON group; ##P < 0.01 vs. STZ group; CON: control group; STZ: streptozotocin group; SH: STZ+sodium hydrosulfide group; SP: STZ+DL-propargylglycine group. (A color version of this figure is available in the online journal.)
Effect of H2S on the NLRP3 inflammasome in DCM
Protein expression levels of NLRP3, activated caspase-1, and ASC were measured to elucidate the activation status of NLRP3 inflammasome. Immunoblotting results exhibited that there was significant activation of NLRP3 inflammasome in the STZ group in contrast to the CON group. Exogenous H2S downregulated the expression levels of NLRP3, caspase-1, and ASC proteins, whereas treatment with PAG significantly upregulated the expression levels of NLRP3 inflammasome-associated proteins compared with diabetic rats (Figure 7). The results exhibited that H2S downregulated the activation status of NLRP3 inflammasome in diabetic cardiomyocytes.
Figure 7.
Effects of H2S on NLRP3 inflammasome expression in rat myocardial tissues of each group. (a) Representative western blotting showing NLRP3, ASC, and caspase-1 (p20) alterations. (b–d) Quantitative analyses of NLRP3, ASC, and caspase-1 (p20) proteins. β-actin was used for normalization of protein expression levels. Values, mean ± SD; n = 7; **P < 0.01 vs. CON group; ##P < 0.01 vs. STZ group; CON: control group; STZ: streptozotocin group; SH: STZ+sodium hydrosulfide group; SP: STZ+DL-propargylglycine group.
Discussion
DCM is a heart disease specifically associated with diabetes mellitus and is mainly characterized by cardiac systolic and diastolic dysfunction.17 Cardiac hypertrophy and myocardial histological and ultrastructural damage are observed in the diabetic heart.18 Among these, myocardial fibrosis is also a key pathological hallmark of DCM.19 In addition, CK and LDH are usually considered as the biochemical markers of myocardial injury.20 Briefly, these indicators are commonly used to assess the degree of diabetic myocardial damage. In the current work, we utilized STZ to establish a type 1 diabetic rat model. Our experimental results exhibited that compared with normal rats, cardiac function deteriorated, whereas the levels of FBG, CK, and LDH increased in diabetic rats. As expected, cardiac hypertrophy, myocardial histological injury, and fibrosis were observed in the diabetic rats, indicating that the animal model of DCM was established successfully.
Hyperglycemia, as the leading characteristic of diabetes mellitus, causes myocardial injury through induction of oxidative stress, inflammation, and even cell death.3 Increased metabolism of high glucose through mitochondrial glucose oxidation contributes to the over-generation of reactive oxygen species (ROS) in diabetes. ROS react with polyunsaturated fatty acids to generate lipid peroxides, and, thus, the content of LPO and MDA reflects the state of oxidative stress injury.21 T-AOC represents the total antioxidant capacity, while some antioxidant enzymes, including GSH-Px, catalase, and SOD, are usually regarded as the indicators of reductive capability in mammals. Furthermore, Thioredoxin is an important antioxidant protein in vivo.22 TXNIP has been reported to promote oxidative stress by combining with and inhibiting Thioredoxin.23 In this rat model of diabetes, the results showed that myocardial T-AOC, the activities of antioxidant enzymes, and Thioredoxin expression were decreased, whereas the levels of MDA, LPO, and TXNIP expression were increased in the diabetic myocardia compared with normal myocardia, which indicated that hyperglycemia caused TXNIP-mediated oxidative stress injury in diabetic cardiomyocytes.
H2S is a novel endogenous gas messenger that plays a crucial role in the various pathophysiological processes in mammalian organisms, such as in the cardiovascular system, nervous system, and urinary system.24–26 H2S has been reported to exert anti-oxidative, anti-inflammatory, and anti-apoptotic activities in diabetic myocardium.27 The concentration of H2S is essential to the exertion of its protective effects in vivo.13 Evidence suggests that NaHS treatment inhibits TXNIP expression, whereas PAG treatment upregulates TXNIP expression in endothelial cells.28 This study found that exogenous H2S significantly alleviated diabetes-induced cardiac hypertrophy and oxidative stress, enhanced cardiac function, ameliorated histomorphological damage, and downregulated cardiac injury enzyme (CK and LDH) levels in the SH group. However, once the generation of endogenous H2S was suppressed with PAG, oxidative stress, cardiac dysfunction and injury, and histomorphological damage were further aggravated in the SP group. The current results exhibited that by improving the balance of redox status in diabetic myocardia, H2S had protective effects against diabetes-induced damage.
Recent evidence reveals that oxidative stress stimulates chronic inflammatory reaction in the diabetic myocardium.29 This inflammatory reaction is one of the most important mechanisms leading to diabetic myocardial damage.30 IL-6 and TNF-α are important proinflammatory mediators in vivo.31 This work showed that infiltration by inflammatory cells and levels of IL-6 and TNF-α were increased in the STZ group, which indicated that inflammation existed in the diabetic myocardium. After treatment with NaHS, the inflammatory reaction was reduced remarkably in the SH group. However, in the SP group, the inflammatory reaction was further aggravated in contrast to the STZ group, which demonstrated that H2S exerted an anti-inflammatory role in diabetic cardiomyocytes.
Inflammasomes are involved in the inflammatory process and even cause myocardial injury.32 Among many known inflammasome complexes, the NLRP3 inflammasome complex is well studied and comprises of NLRP3, procaspase-1, and ASC.33 Upon activation of NLRP3, procaspase-1 is cleaved to release active caspase-1, which cleaves proinflammatory mediator precursors, pro-IL-1β and pro-IL-18, into their active forms, further exacerbating the inflammatory process.34 Furthermore, the involvement of TXNIP is important for the regulation of NLRP3 inflammasome.35 Under physiological conditions, TXNIP combines with Thioredoxin and does not interact with NLRP3 protein. Once excessive ROS stimulation occurs, TXNIP is separated from Thioredoxin and then combines with NLRP3 protein, regulating the activity of the NLRP3 inflammasome positively. The NLRP3 inflammasome is a critical contributor to the innate immune response and has become a key regulator of inflammatory reaction in diabetes mellitus.36 Inhibition of NLRP3 inflammasome activation significantly alleviated myocardial injury in a rat model of DCM.37 This study found that compared with normal rats, there was decreased expression level of Thioredoxin and increased expression levels of TXNIP and NLRP3 inflammasome-related proteins in diabetic hearts. After treating the diabetic rats with exogenous H2S, the protein expression level of Thioredoxin was raised, whereas the expression of TXNIP and NLRP3 inflammasome complex-associated proteins was reduced in myocardial tissue in the SH group. On the other hand, when the generation of endogenous H2S was suppressed by PAG, the above-mentioned indicators deteriorated in the SP group, further demonstrating that the concentration of H2S was important for the exertion of its protective effects. The current work indicated that H2S exerted an anti-inflammatory role in diabetic myocardia by downregulation of TXNIP-mediated NLRP3 inflammasome activation.
In conclusion, H2S alleviated hyperglycemia-mediated myocardial inflammation in type 1 diabetes. As illustrated in Figure 8, the mechanism may involve the inhibition of TXNIP-mediated NLRP3 inflammasome activation. This evidence suggests that strategies aimed at inhibiting TXNIP-mediated NLRP3 inflammasome by use of exogenous H2S might serve as efficient targeted therapy in diabetic cardiomyocytes.
Figure 8.
Possible mechanism of H2S against TXNIP-mediated NLRP3 inflammasome activation in diabetic cardiomyopathy. (A color version of this figure is available in the online journal.)
Authors’ contributions
QJ, SM, XL, SM, and RY designed the experiments, carried out the studies, participated in collecting data, and drafted the manuscript. QJ, XL, and SM analyzed the statistical results. SM and RY revised the manuscript.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Natural Science Research Project of the Education Commission of Anhui Province (grant number: KJ2018A0994); the Science and Technology Development Research Project of Bengbu Medical College (grant numbers: BYKF1706, BYKF17114); and the Training Programs of Innovation and Entrepreneurship for Undergraduates (grant numbers: 201710367012, 201810367022), China.
ORCID iD
References
- 1.Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev 2013; 93:137–88 [DOI] [PubMed] [Google Scholar]
- 2.Chen J, Zhang Z, Cai L. Diabetic cardiomyopathy and its prevention by nrf2: current status. Diabetes Metab J 2014; 38:337–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bugger H, Abel ED. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia 2014; 57:660–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res 2018; 122:624–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee H, Lim Y. Gamma-tocopherol ameliorates hyperglycemia-induced hepatic inflammation associated with NLRP3 inflammasome in alloxan-induced diabetic mice. Nutr Res Pract 2019; 13:377–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vince JE, Silke J. The intersection of cell death and inflammasome activation. Cell Mol Life Sci 2016; 73:2349–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sepehri Z, Kiani Z, Afshari M, Kohan F, Dalvand A, Ghavami S. Inflammasomes and type 2 diabetes: an updated systematic review. Immunol Lett 2017; 192:97–103 [DOI] [PubMed] [Google Scholar]
- 8.Spindel ON, World C, Berk BC. Thioredoxin interacting protein: redox dependent and independent regulatory mechanisms. Antioxid Redox Signal 2012; 16:587–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol 2010; 11:136–40 [DOI] [PubMed] [Google Scholar]
- 10.Yang F, Qin Y, Wang Y, Meng S, Xian H, Che H, Lv J, Li Y, Yu Y, Bai Y, Wang L. Metformin inhibits the NLRP3 inflammasome via AMPK/mTOR-dependent effects in diabetic cardiomyopathy. Int J Biol Sci 2019; 15:1010–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H, Chen X, Zong B, Yuan H, Wang Z, Wei Y, Wang X, Liu G, Zhang J, Li S, Cheng G, Wang Y, Ma Y. Gypenosides improve diabetic cardiomyopathy by inhibiting ROS-mediated NLRP3 inflammasome activation. J Cell Mol Med 2018; 22:4437–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kabil O, Banerjee R. Redox biochemistry of hydrogen sulfide. J Biol Chem 2010; 285:21903–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang R, Jia Q, Liu XF, Wang YY, Ma SF. Effects of hydrogen sulfide on inducible nitric oxide synthase activity and expression of cardiomyocytes in diabetic rats. Mol Med Rep 2017; 16:5277–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polhemus DJ, Lefer DJ. Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ Res 2014; 114:730–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kar S, Kambis TN, Mishra PK. Hydrogen sulfide-mediated regulation of cell death signaling ameliorates adverse cardiac remodeling and diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol 2019; 316:H1237–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang Z, Zhuang X, Xie C, Hu X, Dong X, Guo Y, Li S, Liao X. Exogenous hydrogen sulfide attenuates high glucose-induced cardiotoxicity by inhibiting NLRP3 inflammasome activation by suppressing TLR4/NF-κB pathway in H9c2 cells. Cell Physiol Biochem 2016; 40:1578–90 [DOI] [PubMed] [Google Scholar]
- 17.Jia G, Whaley-Connell A, Sowers JR. Diabetic cardiomyopathy: a hyperglycaemia- and insulin-resistance-induced heart disease. Diabetologia 2018; 61:21–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohammed Yusof NL, Zainalabidin S, Mohd Fauzi N, Budin SB. Hibiscus sabdariffa (roselle) polyphenol-rich extract averts cardiac functional and structural abnormalities in type 1 diabetic rats. Appl Physiol Nutr Metab 2018; 43:1224–32 [DOI] [PubMed] [Google Scholar]
- 19.Hu X, Bai T, Xu Z, Liu Q, Zheng Y, Cai L. Pathophysiological fundamentals of diabetic cardiomyopathy. Compr Physiol 2017; 7:693–711 [DOI] [PubMed] [Google Scholar]
- 20.Chang G, Chen Y, Zhang H, Zhou W. Trans sodium crocetinate alleviates ischemia/reperfusion-induced myocardial oxidative stress and apoptosis via the SIRT3/FOXO3a/SOD2 signaling pathway. Int Immunopharmacol 2019; 71:361–71 [DOI] [PubMed] [Google Scholar]
- 21.Linhart KB, Glassen K, Peccerella T, Waldherr R, Linhart H, Bartsch H, Seitz HK. The generation of carcinogenic etheno-DNA adducts in the liver of patients with nonalcoholic fatty liver disease. Hepatobiliary Surg Nutr 2015; 4:117–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whayne TF, Jr, Parinandi N, Maulik N. Thioredoxins in cardiovascular disease. Can J Physiol Pharmacol 2015; 93:903–11 [DOI] [PubMed] [Google Scholar]
- 23.Shah A, Xia L, Goldberg H, Lee KW, Quaggin SE, Fantus IG. Thioredoxin-interacting protein mediates high glucose-induced reactive oxygen species generation by mitochondria and the NADPH oxidase, Nox4, in mesangial cells. J Biol Chem 2013; 288:6835–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X, Bian JS. Hydrogen sulfide: a neuromodulator and neuroprotectant in the Central nervous system. ACS Chem Neurosci 2014; 5:876–83 [DOI] [PubMed] [Google Scholar]
- 25.Zhou X, An G, Lu X. Hydrogen sulfide attenuates the development of diabetic cardiomyopathy. Clin Sci (Lond) 2015; 128:325–35 [DOI] [PubMed] [Google Scholar]
- 26.Kasinath BS, Feliers D, Lee HJ. Hydrogen sulfide as a regulatory factor in kidney health and disease. Biochem Pharmacol 2018; 149:29–41 [DOI] [PubMed] [Google Scholar]
- 27.Ye P, Gu Y, Zhu YR, Chao YL, Kong XQ, Luo J, Ren XM, Zuo GF, Zhang DM, Chen SL. Exogenous hydrogen sulfide attenuates the development of diabetic cardiomyopathy via the FoxO1 pathway. J Cell Physiol 2018; 233:9786–98 [DOI] [PubMed] [Google Scholar]
- 28.Tian D, Dong J, Jin S, Teng X, Wu Y. Endogenous hydrogen sulfide-mediated MAPK inhibition preserves endothelial function through TXNIP signaling. Free Radic Biol Med 2017; 110:291–9 [DOI] [PubMed] [Google Scholar]
- 29.Jha JC, Ho F, Dan C, Jandeleit-Dahm K. A causal link between oxidative stress and inflammation in cardiovascular and renal complications of diabetes. Clin Sci (Lond) 2018; 132:1811–36 [DOI] [PubMed] [Google Scholar]
- 30.Palomer X, Pizarro-Delgado J, Vazquez-Carrera M. Emerging actors in diabetic cardiomyopathy: heartbreaker biomarkers or therapeutic targets? Trends Pharmacol Sci 2018; 39:452–67 [DOI] [PubMed] [Google Scholar]
- 31.Jia Q, Yang R, Ma SF, Liu XF. Protective effect of genistein on diaphragm injury in diabetic rats. Lat Am J Pharm 2018; 37:2145–53 [Google Scholar]
- 32.Su Q, Li L, Sun Y, Yang H, Ye Z, Zhao J. Effects of the TLR4/Myd88/NF-κB signaling pathway on NLRP3 inflammasome in coronary microembolization-induced myocardial injury. Cell Physiol Biochem 2018; 47:1497–508 [DOI] [PubMed] [Google Scholar]
- 33.Jo EK, Kim JK, Shin DM, Sasakawa C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol 2016; 13:148–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malik A, Kanneganti TD. Inflammasome activation and assembly at a glance. J Cell Sci 2017; 130:3955–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang C, Xia W, Liu X, Lin J, Wu A. Role of TXNIP/NLRP3 in sepsis-induced myocardial dysfunction. Int J Mol Med 2019; 44:417–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qiu YY, Tang LQ. Roles of the NLRP3 inflammasome in the pathogenesis of diabetic nephropathy. Pharmacol Res 2016; 114:251–64 [DOI] [PubMed] [Google Scholar]
- 37.Li X, Ke X, Li Z, Li B. Vaspin prevents myocardial injury in rats model of diabetic cardiomyopathy by enhancing autophagy and inhibiting inflammaion. Biochem Biophys Res Commun 2019; 514:1–8 [DOI] [PubMed] [Google Scholar]