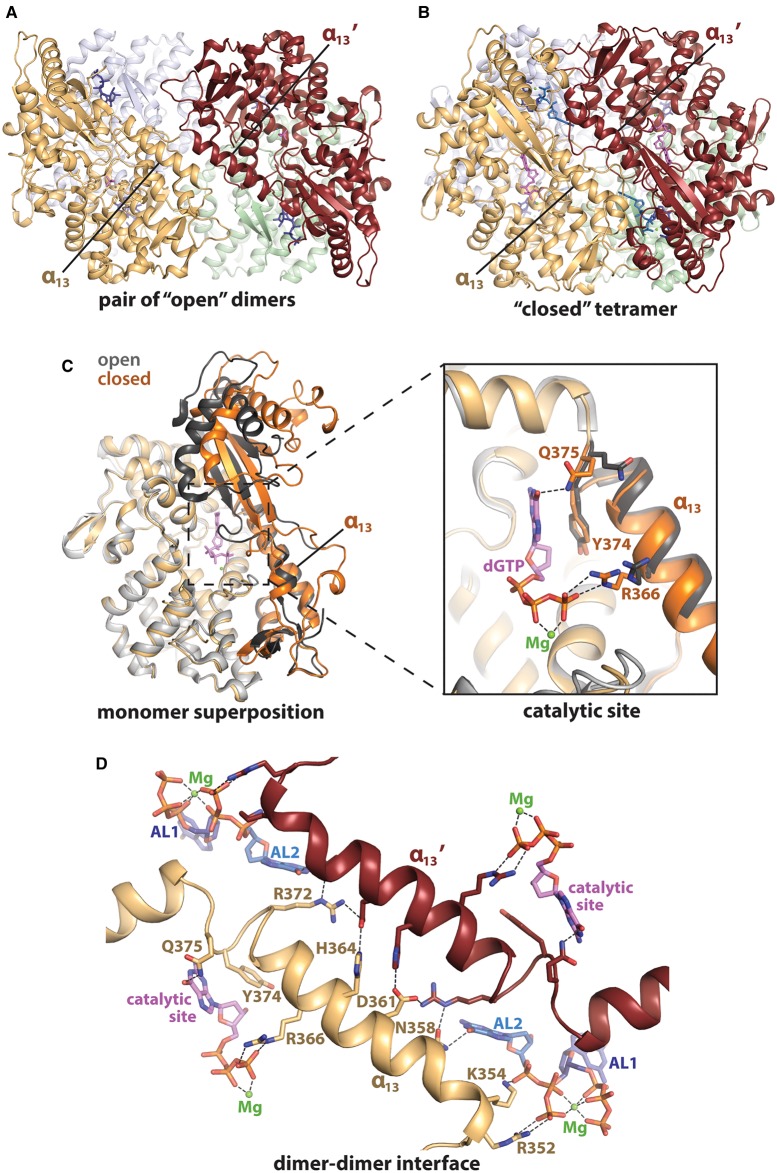
Figure 5. ‘Open’ and ‘closed’ conformations of HD domain.
(A) X-ray crystal structure of the HD domain in ‘open’ conformation co-ordinated to GTP (dark blue) in AL1 (PDB: 4RXO) [95]. The tetramer comprises two dimers, with one formed from the orange and grey monomers, and the second from the maroon and light green monomers. (B) X-ray crystal structure of HD domain in ‘closed’ tetrameric conformation, with dGTP co-ordinated in AL1, AL2 and the catalytic site (PDB: 4BZB) [85]. dGTP is shown in dark blue (AL1), pale blue (AL2) or pink (catalytic site) and magnesium ions are shown as green spheres. (C) Superposition of an HD domain monomer in ‘open’ (grey) and ‘closed’ (orange) conformations. Residues 326–375 and 454–599 differ in their conformation between the ‘open’ and ‘closed’ states and are represented by darker shades of grey and orange, respectively. The dGTP- and magnesium-coordination observed in the catalytic site of the ‘closed’ state is displayed, while the manganese- and phosphate-coordination observed in the ‘open’ state is not shown. (D) Interactions formed by the α13 helix at the dimer–dimer interface (residues Asn358, Asp361, His364 and Arg372), in the catalytic site (Arg366, Tyr374 and Gln375) and in AL2 (Arg352, Lys354 and Asn358). One monomer is shown in pale orange, and its neighbouring monomer at the dimer–dimer interface is shown in maroon. dGTP in AL1 (dark blue), AL2 (pale blue) and the catalytic site (pink) is shown in stick representation. Magnesium ions are represented by green spheres. Hydrogen bonds are shown as dashed lines.