6.
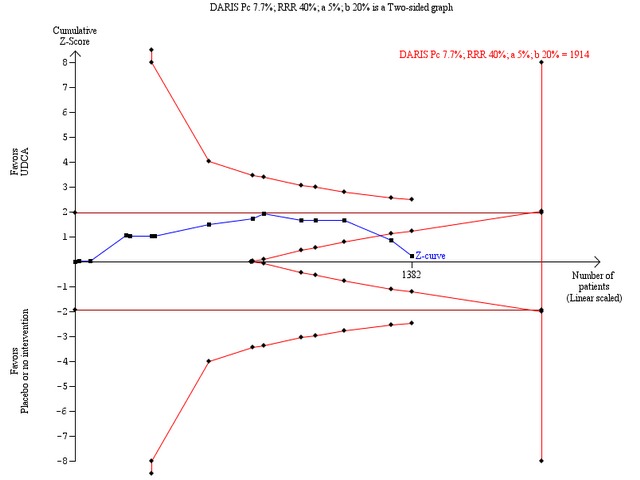
Trial sequential analysis of the random‐effects meta‐analysis of the effect of ursodeoxycholic acid versus placebo or no intervention on all‐cause mortality. The trial sequential analysis is performed with an assumed control proportion of death of 7.7%, an anticipated relative risk reduction (RRR) of 40%, a type 1 error risk of 5% (two‐sided) (a), and a power of 80% (type 2 error risk of 20%) (b). The diversity‐adjusted required information size to detect or reject a RRR of 40% with a between trial heterogeneity of 0% is estimated to 1914 patients. The actually accrued number of patients is 1382, which is 72% of the required information size. The blue cumulative Z‐curve does not cross the red trial sequential alpha‐spending monitoring boundaries for benefit or harm. However, the boundaries for futility (the red inner wedge boundaries showing the trial sequential beta‐spending monitoring boundaries) are crossed. The red conventional boundaries (horizontal line at Z = 1.96 and Z = ‐1.96) for harm or benefit are not crossed. Therefore, there is no evidence to support ursodeoxycholic acid and we can refute that ursodeoxycholic acid influences mortality by a 40% RRR of mortality with the chosen error risks.
